[Behandlung des Fibromyalgiesyndroms – eine interdisziplinäre S3-Leitlinie]
Winfried Häuser 1,2Bernhard Arnold 3,4
Wolfgang Eich 5,6
Eva Felde 7
Christl Flügge 8
Peter Henningsen 9,10
Markus Herrmann 11,12
Volker Köllner 13,14
Edeltraud Kühn 15
Detlev Nutzinger 16,17
Martin Offenbächer 18,19
Marcus Schiltenwolf 20,21
Claudia Sommer 22,23
Kati Thieme 24,25
Ina Kopp 26,27
1 Department of Internal Medicine I, Klinikum Saarbrücken, Germany
2 German Interdisciplinary Association of Pain Therapy DIVS
3 Pain clinic, Klinikum Dachau, Germany
4 German Association for the Study of Pain DGSS
5 Department Internal Medicine II, University Clinics of Heidelberg, Germany
6 German Society of Rheumatology DGRh
7 German Fibromyalgia Association, Seckach, Germany
8 Central Association of Physiotherapy ZVK, Köln, Germany
9 Department of Psychosomatic Medicine and Psychotherapy, Technical University Munich, München, Germany
10 German College of Psychosomatic Medicine DKPM
11 Institute of General Medicine, Otto-von-Guericke-Universität Magdeburg, Germany
12 German Society of General and Family Medicine DEGAM
13 Department of Psychosomatic Medicine, Bliestal Clinics, Blieskastel, Germany
14 German Society of Psychosomatic Medicine and Medical Psychotherapy DGPM
15 German League of People with Arthritis and Rheumatism, Bonn, Germany
16 Medical-psychosomatic clinic, Bad Bramstedt, Germany
17 German Society of Psychiatry, Psychotherapy and Neurology DGPPN
18 Generation Research Program, Ludwig-Maximilian-University München, Bad Tölz, Germany
19 Deutsche Society of Physical Medicine and Rehabilitation DGPMR
20 Section Pain Therapy, University Clinic of Orthopedics, Heidelberg, Germany
21 German Society of Orthopedics and Orthopedic Surgery DGOOC
22 Department of Neurology, University Würzburg, Germany
23 German Society of Neurology DGN
24 Central Institute of Mental Health, Mannheim, Germany
25 German Society of Psychological Pain Research and Therapy DGPSF
26 Institute of Theoretical Surgery, University Marburg, Germany
27 Association of the Scientific Medical Societies in Germany AWMF
Zusammenfassung
Die Prävalenz des Fibromyalgiesyndroms (FMS) in der allgemeinen Bevölkerung beträgt 1–2%. Aufgrund der mit FMS verbundenen hohen Krankheitskosten und den widersprüchlichen Daten zur Wirksamkeit einzelner Behandlungsformen wurden evidenzbasierte Leitlinien entwickelt, um Ärzten und Patienten einen Entscheidungshilfe zu geben. Bisher war in Europa keine interdisziplinäre evidenzbasierte Leitlinie unter Einschluss von Patienten zum FMS verfügbar.
Deswegen wurde von 13 deutschen medizinischen und psychologischen Fachgesellschaften und zwei Patientenselbsthilfeorganisationen eine Leitlinie zu Therapie des FMS entwickelt. Die Durchführung wurde von der Deutschen Interdisziplinären Vereinigung für Schmerztherapie DIVS und der Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften in Deutschland AWMF koordiniert. Eine systematische Literatursuche aller kontrollierte Studien, systematischer Reviews und Metaanalysen wurde über die Datenbanken Cochrane Library (1993–12/2006), Medline (1980–12/2006), PsychInfo (1966–12/2006) und Scopus (1980–12/ 2006) durchgeführt. Für die Vergabe von Evidenzklassen wurde das System des Oxford-Centre for Evidence Based Medicine verwendet. Für die Vergabe von Empfehlungsgraden wurde die Empfehlungsgraduierung der nationalen Versorgungsleitlinien verwendet. Die Erstellung der Empfehlungen erfolgte in einem mehrstufigen nominalen Gruppenprozess, welcher von einer Vertreterin der AWMF moderiert wurde. Die Leitlinie wurde von den Vorständen der beteiligten Fachgesellschaften begutachtet und genehmigt. Die wissenschaftliche Lang- und Kurzversion der Leitlinie wurde am 25. April 2008 von der AWMF ins Internet gestellt: http://www.uni-duesseldorf.de/AWMF/ll/041-004.htm. Eine Kurzversion für Patienten ist ebenfalls verfügbar: http://www.uni-duesseldorf.de/AWMF/ll/041-004p.htm.
Folgende Therapieverfahren erhielten eine starke Empfehlung: Informationen über Diagnose und Behandlungsmöglichkeiten, patienten-zentrierte Kommunikation, aerobes Ausdauertraining, kognitive und operante Verhaltenstherapie, multimodale Therapie und Amitriptylin. Basierend auf Expertenmeinung wurde eine stufenweise Behandlung des FMS empfohlen: Stufe 1 beinhaltet Diagnosebestätigung und Patientenschulung und/oder Behandlung körperlicher und psychischer Komorbiditäten oder aerobes Ausdauertraining oder kognitive und operante Verhaltenstherapie oder Amitriptylin. Stufe 2 beinhaltet multimodale Therapie. Stufe 3 umfasst die folgenden Behandlungsoptionen: keine weitere Behandlung oder Selbstmanagement (aerobes Ausdauertraining, Stressmanagement) und/oder multimodale Auffrischungstherapie und/oder phamakologische Therapie (Duloxetin oder Fluoxetin oder Pregabalin oder Tramadol/Paracetamol) und/oder psychotherapeutische Verfahren (Hypnotherapie oder therapeutisches Schreiben) oder physikalische Therapie (Balneotherapie oder Ganzkörperwärmetherapie) und/oder komplementäre Therapien (Homöopathie oder vegetarische Kost). Die Wahl der Behandlungsoptionen soll auf gemeinsame Entscheidungsfindung und unter Berücksichtigung der Patientenpräferenzen durchgeführt werden.
Schlüsselwörter
Fibromyalgiesyndrom, systematische Übersicht, Leitlinie, Behandlung
Introduction
According to the criteria of the American College of Rheumatology (ACR), fibromyalgia syndrome (FMS) is defined as chronic widespread pain and tenderness in at least eleven of 18 defined tender points [1]. Population based estimates of the prevalence of FMS range from 0.5% to 5.8% [2]. FMS is frequently associated with fatigue, sleep disorder, other functional somatic syndromes, mental and physical disorders [3], [4], as well as disability and diminished quality of life [5]. FMS patients incur high direct medical costs [6], [7] and consume significant indirect costs (e.g., sick-leave, disability pension) [5], [8]. Effective treatment options are therefore needed for both medical and economic reasons [9].
Despite increased knowledge about FMS, there is currently no cure. The absence of any definitive treatment has resulted in a variety of pharmacological and non-pharmacological treatments being prescribed and used by patients diagnosed with FMS [10], [11]. The results of various treatments have been modest and inconsistent. The large number of patients diagnosed with FMS and the conflicting data on treatment effectiveness has led to the development of a number of attempts to create evidence-based guidelines designed to provide patients and physicians guidance in selecting among the alternatives.
Until now three evidence-based guidelines on the management of FMS were available. The interdisciplinary guideline of the American Pain Society was developed by 13 experts in various pain management disciplines and based on a systematic search of the literature until April 2004 [12]. The recommendations of the European League Against Rheumatism (EULAR) were given by 19 experts, mainly rheumatologists, of 11 European countries, and based on a search of the literature until December 2005 [13]. Most non-pharmacological therapies were not considered by EULAR. The two guideline-groups did not include FMS-patient organizations and did not report how consensus on the recommendations was achieved. Therefore we decided to develop an evidence-based guideline which
- expanded the search of literature until December 2006 including all non-pharmacological therapies,
- included members of patient organizations into the guideline group having equal rights,
- was based on a consensus of medical and psychological scientific societies
- followed defined procedures to reach a consensus on recommendations.
The elaborate German version of the guideline is online available (http://www.uni-duesseldorf.de/AWMF/ll/041-004.htm). Below a summary of the methodology and the recommendations on therapy is given to disseminate the guideline into the German and non-German speaking scientific community.
Methods
Selection of the guideline group
The development of the guideline was initiated and coordinated by the German Interdisciplinary Association of Pain Therapy (DIVS), an umbrella organisation of 19 scientific societies engaged in pain therapy. The Association of the Scientific Medical Societies of Germany (AWMF), the umbrella organisation of 152 scientific medical societies in Germany and the German Society of Gastroenterology DGVS gave methodological support. The steering committee of AWMF consisted of 15 experts from 10 different scientific societies and two patients representing the two largest German FMS self-help organisations. All health professionals engaged in the management of FMS were represented within the steering committee and in the eight work groups. The two chairs of the steering committee had been deputized by two German umbrella organizations, the DIVS (W. Häuser) and the AWMF (I. Kopp). The chair of the DIVS was responsible for the coordination of the search and the analysis of literature. The chair of the AWMF was responsible for the consensus procedures to establish the recommendations. Both chairs were not permitted to vote on the recommendations. Two members from different medical and psychological societies were included in each work group. All members of the steering committee and the work groups had been nominated by the board of directors of the scientific societies engaged in the guideline. Members of the steering committee were selected because of scientific and clinical FMS expertises, members of the work group were selected because of scientific and/or clinical expertise. Furthermore, gender, hierarchical position within the medical system, and experience were equally distributed attributes amongst members of the work groups. Thus the whole guideline group comprised 58 persons.
Financing of the guideline
The guideline was financed by membership fees of the scientific societies and two self-help organisations. One grant was given by a private founder to one self-help organisation. No other support was accepted.
Data sources and study selection
We searched the Cochrane Library (1993–12/2006), Medline (1980–12/2006), PsychInfo (1966–12/2006) and Scopus (1980–12/2006). Meta-analyses, systematic reviews and controlled trials were reviewed. References were consistently checked for any relevant articles. If none of these data sources was available for therapies frequently used in Germany, uncontrolled trials were reviewed and expert opinions were considered [14].
Classification of evidences, recommendations and consensus
The levels of evidence were assigned according to the classification system of the Oxford-Centre for Evidence Based Medicine [15]. Grading of the strengths of recommendations was done according to the German program for disease management guidelines: The grade of recommendation depends on the level of evidence. A strong recommendation is based on a level of evidence class I, a recommendation on a level of evidence class II and an open recommendation on levels of evidence III, IV and V. Negative recommendations are graded accordingly. An up- or downgrading of recommendations is possible depending on the consistency of the results of the studies, the clinical relevance of the outcomes and effect sizes of the studies, the benefit-harm ratio, ethical considerations, patients’ preferences and the applicability of the therapies [16]. We downgraded recommendations if only one controlled study of the treatment option was available. We defined “consistent” if at least two controlled studies with similar results were available or in case of > two studies the majority (≥75%) of studies had the same results. Standardized procedures to reach a consensus on recommendations were used. To reach consensus on the final version of the guideline, five versions of the recommendations were developed and discussed in three Delphi rounds, one online-voting and two meetings, where the Nominal Group Technique was applied. The strength of consensus was classified as follows: Strong consensus: consent of >95%, consensus: consent of 75–95%, majority consent: consent of 50–75% and no consent: consent of <50% of the participants. A minority vote with a substantial rationale was possible.
The guideline was reviewed by the board of directors of the societies engaged in the development of the guideline. The guideline was published online by the AWMF on april 25, 2008.
Clinical algorithms
Based on the recommendations a clinical algorithm for a graded therapy of FMS was developed by one chair of the steering committee and modified and consented by the steering committee. A clinical algorithm structural analysis was performed by Dr. Sitter, Institute of Theoretical Surgery Marburg with the Software ALGO [17].
Results
Recommendations
The recommendations for the management of FMS in children and adolescents are not reported here. An overview on the recommendations is given in the Tables 1–4 [Tab. 1], [Tab. 2], [Tab. 3], [Tab. 4]. In the following, we outline the reasons for up- and downgrading. Furthermore we point out, if there is evidence of the efficacy of treatment options after the end of therapy.
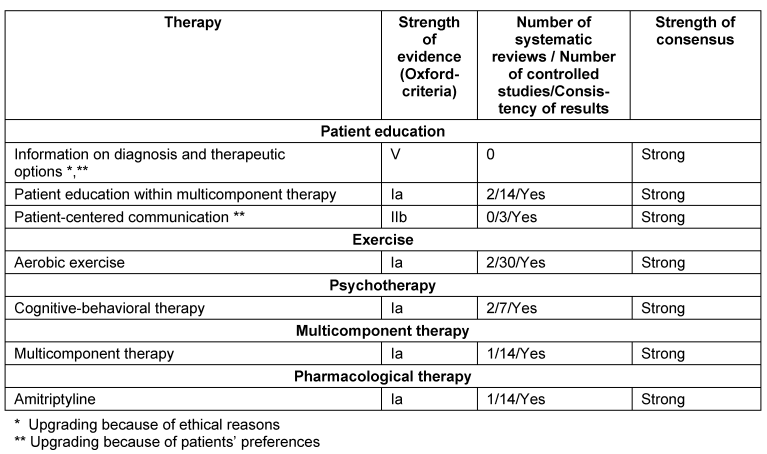
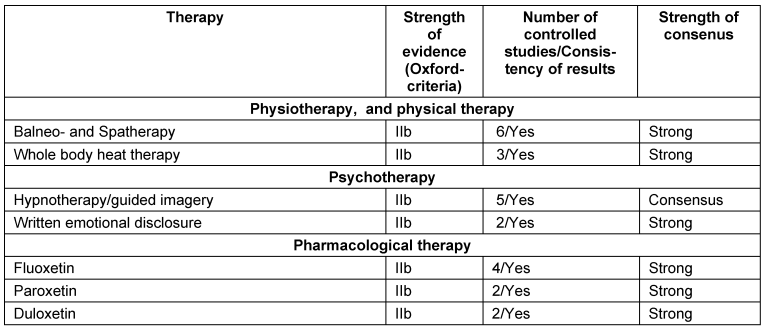
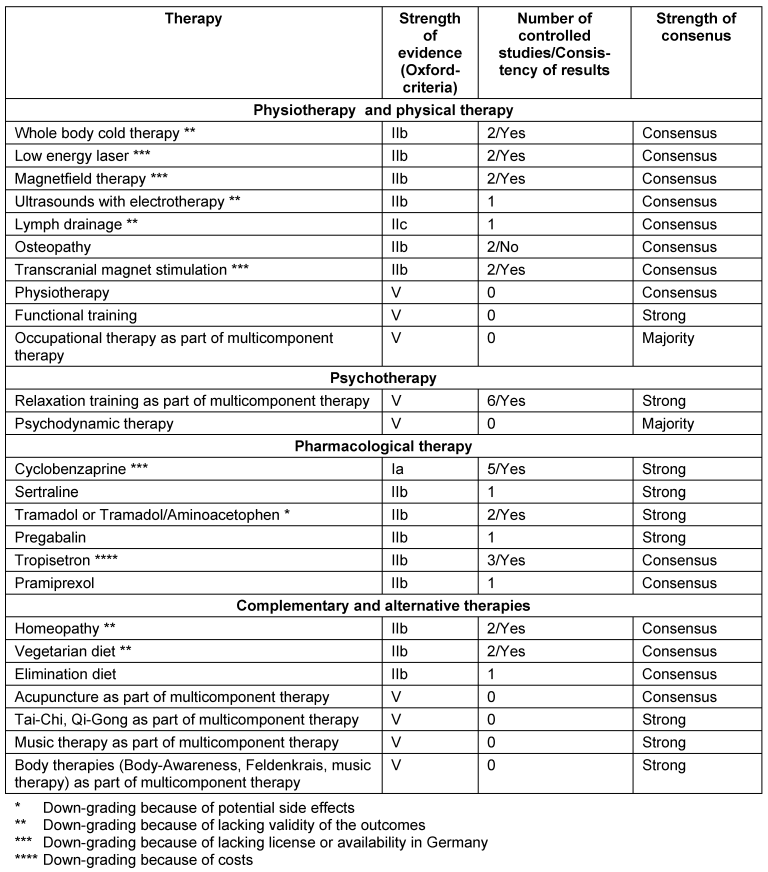
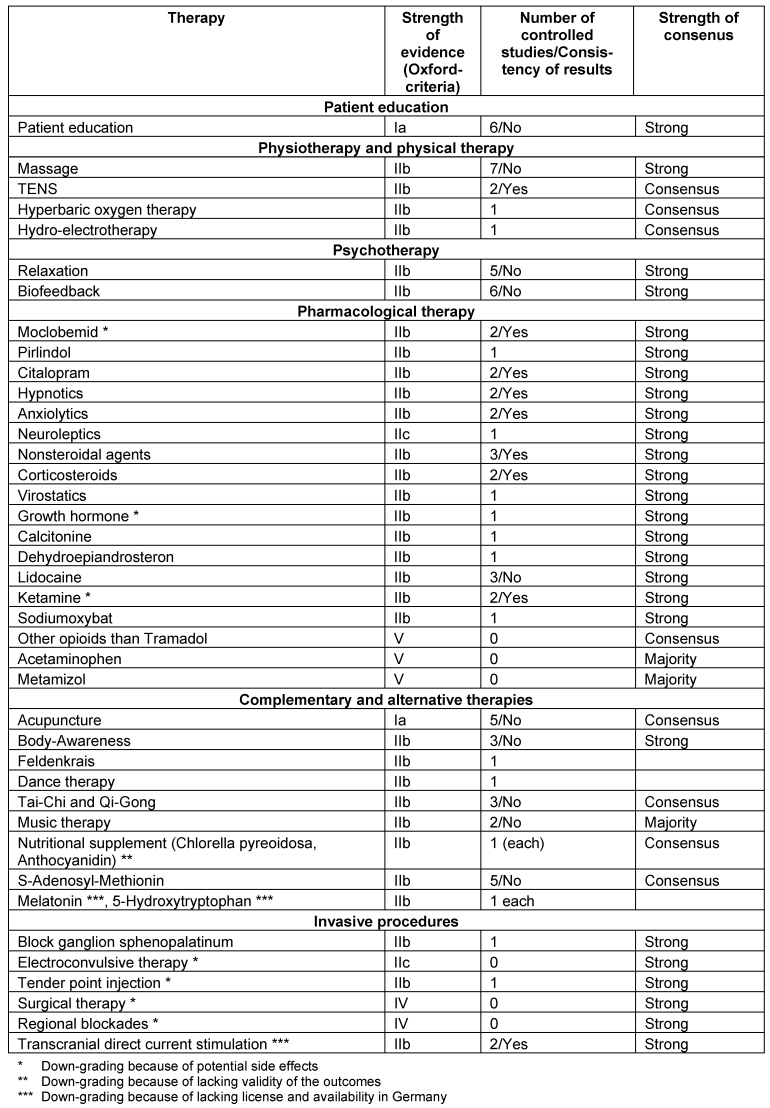
General principles of therapy
Realistic aims of therapy should be developed with the patient. The primary aims of the therapy are improvement of functioning and adaptation to the symptoms. The choice of treatment options should be based on informed decision-making and respect of the patients’ preferences. Furthermore the local availability and cost of treatment options as well as potential physical and mental comorbidities of the patient should be considered. The benefits and risks of any kind of therapy should be monitored regularly by the patient and the physician. Any kind of therapy should only be continued if the benefits outweigh the side effects. The recommendations are in favour of active treatment options such as aerobic exercise and psychological therapies because these modalities had been proven to be superior to standard medical treatment or treatment as usual. Moreover there is evidence that some positive effects of active treatments persist after the end of therapy. These active treatments can be maintained after the end of a therapy programme by the patient without medical assistance [18], [19], [20], [21]. In contrast there is only evidence of a superiority of some pharmacological treatments over placebo with a maximum length of study duration of six months. A continuous pharmacological treatment can only be considered if an ongoing benefit can be detected by regular checks by the patient and physician. A drug holiday after six months pharmacological treatment is a further option [22].
Information on diagnosis
The upgrading into a very strong recommendation was substantiated by two arguments: From the patients’ point of view the diagnosis of FMS often explains a long lasting history of puzzling symptoms and frustrating odyssey through the medical care system. From a physician’s point of view there is an ethical obligation to inform a patient on a diagnosis and its treatment options [18].
Education
Because patient education was part of most studies of multicomponent treatment, patient education as an integrated part of MT was strongly recommended [18].
Patient-centered communication
An upgrading was performed because of patients’ preferences [18].
Aerobic exercise
14/30 studies performed a follow-up. 13/14 studies reported a sustained, but declining reduction of physical symptoms [19].
Psychotherapy
Cognitive behavioural therapy (CBT): Nine of 14 studies performed a follow-up. Five studies reported positive effects in some outcomes at follow-up.
Relaxation training: Because relaxation training was part of some effective MT- or CBT-programs, an open recommendation was given to use relaxation training as an integrated part of MT.
Psychodynamic therapy: Because systematic reviews demonstrated the efficacy of psychodynamic therapy in somatoform pain disorders, an open recommendation was given for FMS-patients who also fulfil the criteria of a somatoform pain disorder [20].
Physiotherapy and physical therapy
Massage, lymph drainage, osteopathy: An upgrading was done because of patients’ preferences within MT.
Whole body cold therapy: The recommendation was downgraded because of the lacking validity of the outcomes.
Low energy laser and magnet field therapy: The recommendations were downgraded, because the therapies are not implemented into medical care in Germany [19].
Pharmacological therapy
Tricyclic antidepressants, serotonin-reuptake inhibitors, noradrenaline-serotonine-reuptake inhibitors: We found consistent positive results of amitriptyline, fluoxetine, paroxetine and duloxetine. We found consistent negative results of citalopram.
Monoaminooxidase inhibitors: The recommendation was downgraded because fatigue and sleeping difficulties increased.
Cyclobenzaprine: The recommendation was downgraded because the drug is not licensed in most European countries.
HT3-receptorantagonists: The recommendation of tropisetron was downgraded because of the costs.
Tramadol, tramadol/aminoacetophen, ketamine, lidocaine: The recommendations were downgraded because of the potential risks [22].
Multicomponent therapy
MT should include at least one psychological and one exercise treatment. 13/14 studies performed follow-ups of which 10 studies demonstrated positive effects of MT [21].
Complementary and alternative therapies
Acupuncture: A systematic review concluded that acupuncture is not effective [23]. A minority vote considered electroacupuncture as effective and that it should be offered. Because of patients’ preferences an open recommendation was given within a MT-treatment.
Homeopathy, vegetarian and elimination diet, nutritional supplements: The recommendations were downgraded because of the lacking validity of the outcomes.
Melatonin, 5-Hydroxytryptophan, S’Adenosyl-Methionin: The recommendations were downgraded because of the lacking license in Germany.
Body-awareness, Feldenkrais, Tai-Chi or Qi Gong, music therapy: Because of patients’ preferences an open recommendation was given within MT [24].
Invasive procedures
Tender point injections and electroconvulsive therapy: Because of the potential harms the recommendation was downgraded.
Transcranial magnet stimulation: Because this treatment option is not available in routine German medical care, the recommendation was downgraded.
Blockades: Single epidural and regional sympathetic blockades were used in pathophysiological observational studies. Because of the potential side effects of blockades an open non-recommendation was given.
Surgical therapy: One author reported long-lasting pain relief after microsurgical release of adhesions at acupuncture points in a case-series. There are potential harms of the surgery. Therefore a strong non-recommendation was given [19].
A stepwise management
A stepwise management approach is outlined in Figure 1 [Fig. 1].
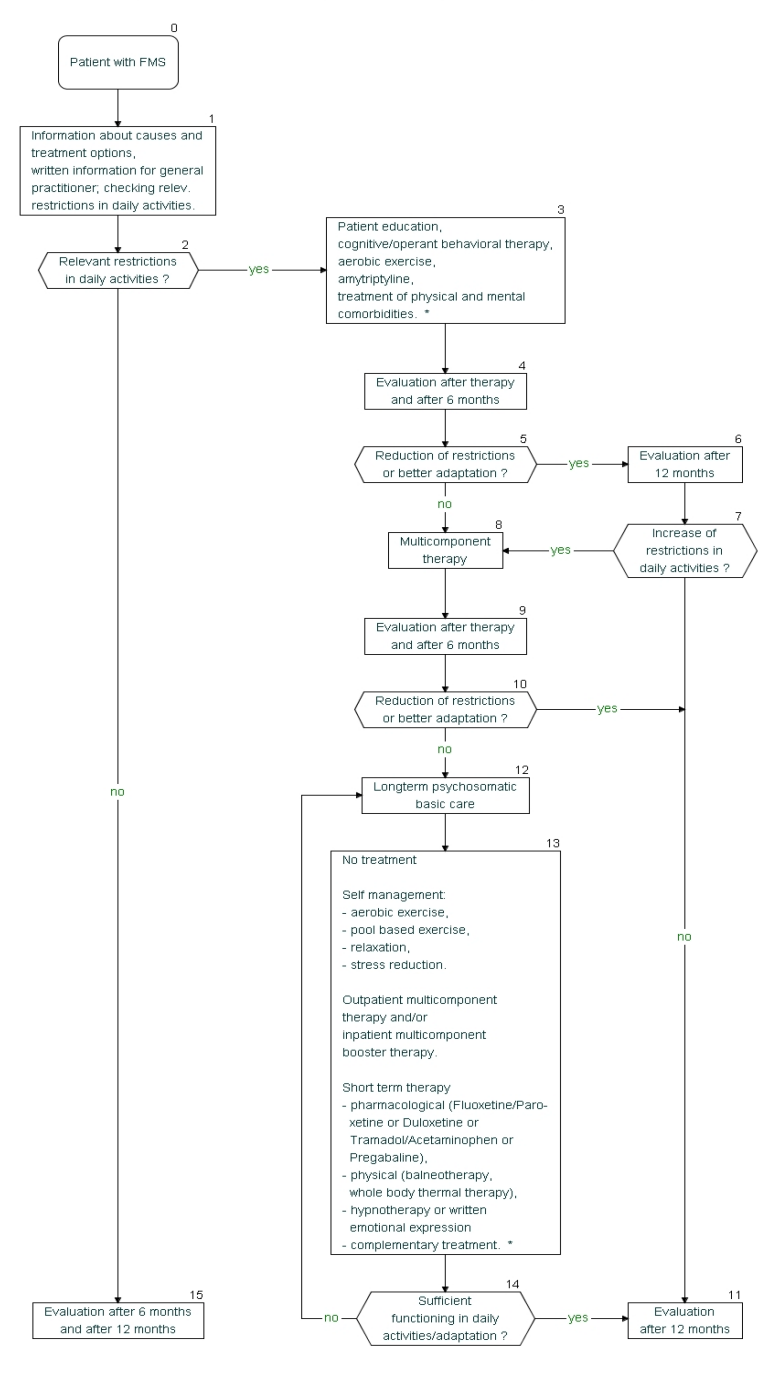
The stepwise approach is based on the levels of evidence and recommendation and costs. The first line therapies had the highest levels of evidence and recommendation. Multicomponent therapy recommended as second line therapy is more costly than the first line therapies. The time frames provided for decision making can be modified depending on the individual situation [18].
Discussion
13 scientific societies and two patient self-help organisations developed a guideline on the management of FMS based on evidence and formal consensus. They met the following obstacles in giving evidence-based recommendations:
- The symptoms and disabilities persist in most adult FMS-patients over time. There is strong evidence for the limited efficacy of some (non-)pharmacological therapies. Most studies reviewed lasted only 4–12 weeks. Follow-ups are only available for aerobic exercise, CBT and MT revealing declining positive effects. The question which therapies should be offered over which period, cannot be answered by the studies available.
- All studies included mainly middle-aged Caucasian women and excluded patients with severe physical and mental disorders. The question how children and adolescents, males, non-Caucasians and patients with severe comorbidities should be managed, cannot be answered by the studies reviewed.
Considering the controversies between somatic and psychosocial approaches in FMS-therapy, the consensus reached in the guideline is remarkable. Some recommendations of the guideline are influenced by a national perspective such as down-grading of recommendations because of lacking license or lack of implementation into medical care in Germany and specific opportunities of the German health system such as rehabilitation medicine. Nevertheless the guideline group hopes that the recommendations will be useful for patients and health care providers in other countries.
The following steps to distribute the recommendations of the guidelines had been done. Short versions of the guideline had been published in some members' journals of the societies involved [25]. Symposia on FMS and the German guideline took place on scientific congresses of the societies involved. The German Society of General and Family Medicine is developing a pocket version of the guideline, which will be evaluated. The guideline was presented on patient-physician seminaries with regional self-help organisations in several German cities (Köln, München, Saarbrücken). The short version of the guideline for patients was published in the members' journals and is also available on the homepages of the self-help organisations involved (http://www.rheuma-liga.de; http://www.fibromyalgie-fms.de/). The guideline will be completely revised in march 2011. Meanwhile new studies on the management of FMS are continuously analysed. Appropriate to requirements earlier revisions of the guideline are possible.
Notes
Conflicts of interest
Financial disclosure of the authors and the steering committee of the guideline at the time of the publication of the guideline: Dr. Eich received research supports from Ergonex. Dr. Schiltenwolf received consulting fees by Pfizer. Dr. Sommer and Dr. Häuser received consulting fees by Elli Lilly and Pfizer. None of the other authors received grants, research support or expert testimonies nor served on the speakers bureau or on the scientific advisory board of pharmaceutical companies. None of the authors was employed by pharmaceutical companies. None of the authors has stock ownerships or options, grants or patents received or pending royalties.
References
[1] Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160-72. DOI: 10.1002/art.1780330203[2] Croft PR. The epidemiology of chronic widespread pain. J Musculoskel Pain. 2002;10(1-2):1-199.
[3] Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65(4):528-33. DOI: 10.1097/01.PSY.0000075977.90337.E7
[4] Van Houdenhove B, Luyten P. Stress, depression and fibromyalgia. Acta Neurol Belg. 2006;106(4):149-56.
[5] Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, Russell IJ, Yunus MB. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis Rheum. 1997;40(9):1560-70. DOI: 10.1002/art.1780400904
[6] Penrod JR, Bernatsky S, Adam V, Baron M, Dayan N, Dobkin PL. Health services costs and their determinants in women with fibromyalgia. J Rheumatol. 2004;31(7):1391-8.
[7] White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: direct health care costs of fibromyalgia syndrome in London, Canada. J Rheumatol. 1999;26(4):885-9.
[8] Henriksson CM, Liedberg GM, Gerdle B. Women with fibromyalgia: work and rehabilitation. Disabil Rehabil. 2005;27(12):685-94. DOI: 10.1080/09638280400009089
[9] Robinson RL, Jones ML. In search of pharmacoeconomic evaluations for fibromyalgia treatments: a review. Expert Opin Pharmacother. 2006;7(8):1027-39. DOI: 10.1517/14656566.7.8.1027
[10] Müller A, Hartmann M, Eich W. Inanspruchnahme medizinischer Versorgungsleistungen. Untersuchung bei Patienten mit Fibromyalgiesyndrom (FMS) [Health care utilization in patients with Fibromyalgia Syndrome (FMS)]. Schmerz. 2000;14(2):77-83. DOI: 10.1007/s004820050225
[11] Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. DOI: 10.1186/1471-2474-8-27
[12] Buckhardt CS, Goldenberg D, Crofford L, Gerwin R, Gowens S, Jackson K, Kugel P, McCarberg W, Rudin N, Schanberg L, Taylor AG, Taylor J, Turk D. Guideline for the management of fibromyalgia syndrome pain in adults and children. Glenview (IL): American Pain Society (APS); 2005. (Clinical practice guideline; no. 4).
[13] Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, Da Silva JA, Danneskiold-Samsøe B, Dincer F, Henriksson C, Henriksson KG, Kosek E, Longley K, McCarthy GM, Perrot S, Puszczewicz M, Sarzi-Puttini P, Silman A, Späth M, Choy EH; EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536-41. DOI: 10.1136/ard.2007.071522
[14] Bernardy K, Klose P, Uçeyler N, Kopp I, Häuser W. Methodische Grundlagen für die Entwicklung der Leitlinienempfehlungen (Methodenreport). [Methodological fundamentals for the development of the guideline]. Schmerz. 2008;22(3):244-54. DOI: 10.1007/s00482-008-0670-8
[15] Oxford Centre for Evidence-based Medicine. Levels of Evidence and Grades of Recommendation. Oxford: CEBM; 2001. Available from: http://www.cebm.net/index.aspx?o=1025#levels
[16] Ollenschläger G, Kopp I, Lelgemann M, Sänger S, Heymans L, Thole H, Trapp H, Lorenz W, Selbmann HK, Encke A. Nationale Versorgungsleitlinien von BAK, AWMF und KBV. Hintergrund, Methodik und Instrumente [The German program for disease management guidelines. Background, methods, and development process]. Med Klin (Munich). 2006;101(10):840-5. DOI: 10.1007/s00063-006-1114-9
[17] Sitter H, Prünte H, Lorenz W. A new version of the programme ALGO for clinical algorithms. In: Brender J, Christensen JP, Scherrer JR, McNair P, Hrsg. Medical informatics Europe '96. Amsterdam: IOS Press; 1998. p. 654-7. (Studies in health technology and informatics; 34).
[18] Klement A, Häuser W, Brückle W, Eidmann U, Felde E, Herrmann M, Kühn-Becker H, Offenbächer M, Settan M, Schiltenwolf M, von Wachter M, Eich W. Allgemeine Behandlungsgrundsatze, Versorgungskoordination und Patientenschulung beim Fibromyalgiesyndrom und chronischen Schmerzen in mehreren Körperregionen [Principles of treatment, coordination of medical care and patient education in fibromyalgia syndrome and chronic widespread pain]. Schmerz. 2008;22(3):283-94. DOI: 10.1007/s00482-008-0673-5
[19] Schiltenwolf M, Häuser W, Felde E, Flügge C, Häfner R, Settan M, Offenbächer M. Physiotherapie, medizinische Trainingstherapie und physikalische Therapie beim Fibromyalgiesyndrom [Physiotherapy, exercise and strength training and physical therapies in the treatment of fibromyalgia syndrome]. Schmerz. 2008;22(3):303-12. DOI: 10.1007/s00482-008-0675-3
[20] Thieme K, Häuser W, Batra A, Bernardy K, Felde E, Gesmann M, Illhardt A, Settan M, Wörz R, Köllner V. Psychotherapie bei Patienten mit Fibromyalgiesyndrom [Psychotherapy in patients with fibromyalgia syndrome]. Schmerz. 2008;22(3):295-302. DOI: 10.1007/s00482-008-0674-4
[21] Arnold B, Häuser W, Bernardy K, Brückle W, Friedel E, Köllner V, Kühn-Becker H, Richter M, Weigl M, Weiss T, Offenbächer M. Multimodale Therapie des Fibromyalgiesyndroms [Multicomponent therapy for treatment of fibromyalgia syndrome]. Schmerz. 2008;22(3):334-8. DOI: 10.1007/s00482-008-0678-0
[22] Sommer C, Häuser W, Berliner M, Brückle W, Ehlers S, Mönkemöller K, Moradi B, Petzke F, Uçeyler N, Wörz R, Winter E, Nutzinger DO. Medikamentöse Therapie des Fibromyalgiesyndroms [Pharmacological treatment of fibromyalgia syndrome]. Schmerz. 2008;22(3):313-23. DOI: 10.1007/s00482-008-0676-2
[23] Mayhew E, Ernst E. Acupuncture for fibromyalgia--a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46(5):801-4. DOI: 10.1093/rheumatology/kel406
[24] Langhorst J, Häuser W, Irnich D, Speeck N, Felde E, Winkelmann A, Lucius H, Michalsen A, Musial F. Komplementäre und alternative Verfahren beim Fibromyalgiesyndrom [Alternative and complementary therapies in fibromyalgia syndrome]. Schmerz. 2008;22(3):324-33. DOI: 10.1007/s00482-008-0677-1
[25] Köllner V, Bernardy K, Häuser W. Fibromyalgiesyndrom - Empfehlungen für die ärztliche psychosomatische und psychotherapeutische Versorgung - Die interdisziplinäre S3-Leitlinie "Definition, Pathophysiologie, Diagnostik und Therapie des Fibromyalgiesydroms" [Fibromyalgia syndrome - recommendations of the interdisciplinary guideline "Definition, pathophysiology, diagnosis and therapy of fibromyalgia syndrome (FMS)"]. Ärztl Psychother Psychosom Med. 2008;3(3):166-72.




