The use of antiviral Phthalocyanine mouthwash as a preventive measure against COVID-19
Fabiano Vieira Vilhena 1Verônica Caroline Brito Reia 2
Bernardo da Fonseca Orcina 2
Caíque Andrade Santos 2
Mariana Zangrando 2
Rodrigo Cardoso de Oliveira 2
Paulo Sérgio da Silva Santos 2
1 TRIALS – Oral Health & Technologies, Bauru, São Paulo, Brazil
2 Bauru School of Dentistry, University of São Paulo, Bauru, São Paulo, Brazil
Letter to the editor
Vaccines and other drugs have been developed and delivered to fight SARS-CoV-2. Successful measures to prevent infection with the virus, such as wearing masks, attention to hygiene and social distancing in conjunction with antiviral mouthwashes, have been recommended to the population for daily use during the COVID-19 pandemic [1], [2].
Scientific evidence has shown the presence of viruses in oral structures and indications for antiviral oral antiseptics have been investigated. Thus, in vitro studies with mouthwashes against coronavirus published during the pandemic seemed promising [3]. However, until September 2020 there was no clinical or laboratorial evidence for their indication, since only few studies had been published [4]. In March 2021 (Ather et al. 2021 [5]), based on the available evidence. It was demonstrated that the use of antiviral oral antiseptics (mouthrinses) had the potential to reduce the in vitro viral load of SARS-CoV-2, but the in vivo effectiveness was still inconclusive [5]. Only in April 2021 was it suggested that there was sufficient in vitro and in vivo evidence of the effectiveness of some oral antiseptics in inactivating SARS-CoV-2 and other coronaviruses [6] (Figure 1 [Fig. 1]).
Figure 1: Timeline of scientific evidence for antiviral mouthwash recommendation against SARS-CoV-2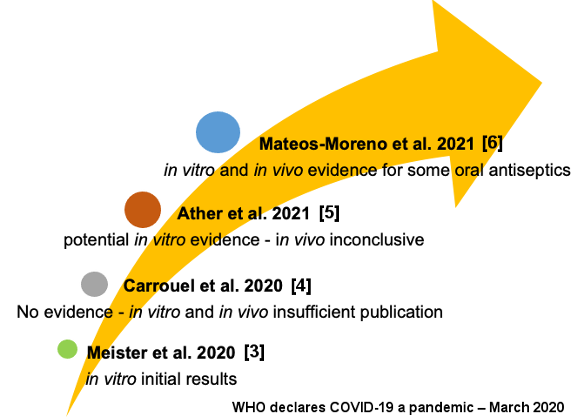
Our research group reinforces the claims for the potential of antiviral mouthwashes to combat COVID-19. Recently, an in vitro evaluation of the virucidal activity of a mouthwash containing antiviral phthalocyanine derivative (APD), a substance that can promote reactive oxygen species generation or redox processes [7], was conducted according to the TCID50 methodology (Median Tissue Culture Infectious Dose). The periods of 30 seconds, 1 minute, and 5 minutes were observed, reaching a percentage of viral inactivation above 99.9% of the SARS-CoV-2 viral load, as shown in Table 1 [Tab. 1]. This research was approved by Human Research Ethics Committee (CAAE 34070620.6.0000.5417) of the institution that performed the research.
Table 1: Percentage of viral inactivation of APD Mouthwash
This is another relevant result of the positive action of APD, since a reduction in the viral load of SARS-CoV-2 above 90% has already been demonstrated in previous in vitro studies [8], [9], as well as the rapid clinical improvement of COVID-19 symptoms [10]. In a randomized study conducted in a hospital environment, COVID-19 patients with mild and moderate cases underwent an adjuvant APD mouthwash protocol. The APD mouthwash group, with a maximum of 7 days of symptoms associated with conventional treatment for COVID-19, was discharged sooner (4 days median). This group also presented lower disease severity (without intensive care unit [ICU] or deaths) when compared to the non-APD mouthwash group (7 days median, 28.6% ICU, 14.3% mortality rate) [8].
In short, the prospect of a low-cost, hygienic personal-care method, suggestive of good option such as the use of antiviral oral antiseptic, may be an important strategy for reducing the impact of COVID-19.
Notes
Competing interests
All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Vilhena reports personal fees from TRIALS Inc while conducting the study. In addition, Dr. Vilhena has a patent pending. Dr. DA Silva Santos reports grants from CNPq process nº. 309525/2018-7. The other authors claim no conflicts of interest
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (Finance Code 001).
Acknowledgments
CROP Biotechnology for the experimental design and execution.
References
[1] Kramer A, Eggers M, Hübner NO, Walger P, Steinmann E, Exner M. Virucidal gargling and virucidal nasal spray. GMS Hyg Infect Control. 2021 Jan 18;16:Doc02. DOI: 10.3205/dgkh000373[2] da Silva Santos PS, da Fonseca Orcina B, da Costa Alves LM, Cardoso de Oliveira R, Mariana Schutzer Ragghianti Zangrando M, Vieira Vilhena F. A Recommendation of PHTALOX® Mouthwash for Preventing Infection and Progression of COVID-19. Acta Scient Dent Sci. 2020;4(12):111-2. DOI: 10.31080/ASDS.2020.04.0991
[3] Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, Groß R, Münch J, Krawczyk A, Steinmann J, Steinmann J, Pfaender S, Steinmann E. Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2. J Infect Dis. 2020 09;222(8):1289-92. DOI: 10.1093/infdis/jiaa471
[4] Carrouel F, Gonçalves LS, Conte MP, Campus G, Fisher J, Fraticelli L, Gadea-Deschamps E, Ottolenghi L, Bourgeois D. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J Dent Res. 2021 Feb;100(2):124-32. DOI: 10.1177/0022034520967933
[5] Ather A, Parolia A, Ruparel NB. Efficacy of Mouth Rinses Against SARS-CoV-2: A Scoping Review. Front Dent Med. 2021;2:648547. DOI: 10.3389/fdmed.2021.648547
[6] Mateos-Moreno MV, Mira A, Ausina-Márquez V, Ferrer MD. Oral antiseptics against coronavirus: in-vitro and clinical evidence. J Hosp Infect. 2021 Jul;113:30-43. DOI: 10.1016/j.jhin.2021.04.004
[7] Teodoro G, Santos C, Carvalho M, Koga-Ito C, Khouri S, Vilhena F. PHTALOX® Antimicrobial Action and Cytotoxicity: in vitro Study. J Dent Res.2020;99(Spec. Iss. A):0839. Available frrom: https://iadr.abstractarchives.com/abstract/20iags-3314903/phtalox-antimicrobial-action-and-cytotoxicity-in-vitro-study
[8] Santos PSS, Orcina BF, Machado RRG, et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19 [Preprint]. Research Square. 2021 Mar 16. DOI: 10.21203/rs.3.rs-330173/v1
[9] Santos C, da Fonseca Orcina B, Brito Reia VC, Ribeiro LG, Grotto RMT, Prudenciatti A, de Moraes LN, Ragghianti Zangrando M, Vilhena FV, da Silva Santos PS. Virucidal Activity of the Antiseptic Mouthwash and Dental Gel Containing Anionic Phthalocyanine Derivative: In vitro Study. Clin Cosmet Investig Dent. 2021;13:269-74. DOI: 10.2147/CCIDE.S315419
[10] da Fonseca Orcina B, Vilhena FV, Cardoso de Oliveira R, Marques da Costa Alves L, Araki K, Toma SH, Ragghianti Zangrando MS, da Silva Santos PS. A Phthalocyanine Derivate Mouthwash to Gargling/Rinsing as an Option to Reduce Clinical Symptoms of COVID-19: Case Series. Clin Cosmet Investig Dent. 2021;13:47-50. DOI: 10.2147/CCIDE.S295423




