[Erster Bericht über Klebsiella pneumoniae Carbapenemase-bildende Pseudomonas aeruginosa-Stämme, isoliert von Verbrennungspatienten im Iran: phenotypische und genotypische Methoden]
Abdolaziz Rastegar Lari 1,2Leila Azimi 1,2
Mohammad Rahbar 3
Reza Alaghehbandan 4
Mahboobeh Sattarzadeh-Tabrizi 5
1 Department of Microbiology and Razi Drug Research Center, Iran University of Medical Sciences, Tehran, Iran
2 Department of Microbiology, Tehran University of Medical Sciences, Tehran, Iran
3 Department of Microbiology, Iranian Reference Health Laboratory, Ministry of Health and Medical Education, Tehran, Iran
4 Department of Pathology and Immunology, Washington University School of Medicine, Barnes Jewish Hospital, St. Louis, MO, USA
5 Motahhari Burn Hospital, Tehran University of Medical Sciences, Tehran, Iran
Zusammenfassung
Wundinfektionen bei Verbrennungspatienten mit Carbapenem-resistentem Pseudomonas (P.) aeruginosa sind ein wachsendes Problem. Einer der hauptsächlichen Resistenzmechanismen gegen Carbapeneme ist die Fähigkeit von P. aeruginosa, Carbapenemase-Enzyme zu bilden. Klebsiella pneumonia Carbapenemase (KPC) ist eine wichtige Carbapenemase, die Carbapeneme hydrolysieren kann. Der modifizierte Hodgetest (MHT) und Boronsäure als KPC-Inhibitor sind zwei phenotypische Methoden zur Detektion der Carbapenemase. Die Sensitivität und Spezifität beider Tests zur KPC-Identifikation kann mittels PCR bestimmt werden.
In der Studie wurden 241 P. aeruginosa-Stämme von Wunden hospitalisierter Verbrennungspatienten isoliert. Carbapenem-resistente P. aeruginosa-Isolate wurden mittels Plättchendiffusionstest bestimmt. KPC-bildende Carbapenem-resistente Stämme wurden mittels modifiziertem Hodgetest (MHT) und anschließend mit Boronsäure detektiert. Stämme mit positiver Reaktion wurden mittels PCR analysiert.
168 Isolate waren resistent gegen Carbapeneme und 75 waren im MHT positiv. Drei Isolate exhibierten mindestens 5-mm Durchmesser als Differenz, wenn Meropenem mit Boronsäure kombiniert wurde vs Meropenem allein im Boronsäuretest. Zwei Stämme hatten eine spezifische Bande mit Primer Nr. 1 in der Gelelektrophorese.
Die Studie zeigt, dass der MHT neben seiner hohen Sensitivität abhängig von der Bakterienspecies eine unterschiedliche Spezifität aufweist. Ferner erhöht der Einsatz eines KPC-Inhibitors wie Boronsäure nicht die Sensitivität und Spezifität bei den isolierten Stämmen. Daher scheint die Sequenzierung mittels PCR der Goldstandard für die Detektion KPC-bildender P. aeruginosa-Stämme zu sein.
Schlüsselwörter
P. aeruginosa, KPC, Boronsäure, modifizierter Hodge-Test, blaKPC
Background
Resistance to carbapenems such as broad spectrum betalactam antibiotics in Pseudomonas aeruginosa is an increasing challenge wordwide [1], [2], [3]. A growing incidence of carbapenem-resistant P. aeruginosa is associated with KPC production, especially in burn patients, and is an important concern in health-care systems [1]. The importance of this resistance is due to the potential for resistance to all betalactam antibiotics in KPC-producing microorganisms, which is one of the main choices for treatment of wound infection [4], [5], [6], [7]. KPC-producing bacteria are emerging in various countries and regions, such as Greece, Iran and Latin America [4], [8], [9]. Rapid and accurate detection of KPC-producing bacteria is necessary for preventing the spread of the kpc gene, given that it is located in transferable genetic elements (i.e. plasmids and transposons) [4], [5], [10]. According to CLSI (Clinical and Laboratory Standards Institute), MHT is one of the phenotypic methods of KPC confirmation ([11], Supplemental Table 2A-S2); however, some studies suggest that MHT may not have a high specificity for the identification of KPC and may only confirm carbapebemase enzymes but not carbapenemase types. Nevertheless, most researchers believe that MHT has a high sensitivity rate [12].
The use of boronic acid – a KPC inhibitor – is another phenotypic method for confirming KPC. Moreover, PCR for the kpc gene is utilized for molecular detection of this enzyme. The aim of this study was to evaluate these two phenotypic methods (MHT and boronic acid) for the detection of KPC by PCR as a molecular test.
Materials and methods
Bacterial strains
In this study, 241 Pseudomonas spp. were isolated from hospitalized burn patients in Motahari Hospital in Tehran, Iran. The species of bacteria were determined by specific biochemical tests such as oxidase, TSI, and gelatinase. PCR was used to confirm identification of Pseudomonas aeruginosa strains using specific primers for oprI for bacteria genus and oprL for species [13]. Pseudomonas aeruginosa ATCC 27853 and Acinetobacter baumannii ATCC 19606 were used as the positive and negative controls, respectively.
Antibiotic susceptibility testing was conducted against carbapenems (Imipenem, Meropenem and Ertapenem) using the disk diffusion method according to the CLSI recommendation. The modified Hodge test was performed for carbapenem-resistant strains.
Modified Hodge Test
According to CLSI, MHT is one of the confirmatory tests for phenotypic identification of the KPC enzyme. In this study, MHT was performed according to the CLSI recommendation by using E. coli ATCC 25922. K. pneumoniae ATCC BAA-1705 – MHT-positive was used as a positive control.
Use of KPC inhibitor
Using 400 µg 3-amino phenyl boronic acid (APBA) as a KPC inhibitor, per disk plus Meropenem vs Meropenem alone is another phenotypic method for confirming identification of KPC. Strains with an increase of at least 5 mm around Meropenem plus APBA vs Meropenem alone were considered KPC-producing strains. On the other hand, the synergistic effect of Meropenem plus Oxacillin (750 µg/disc) was used to eliminate false positive responses.
PCR of the kpc gene
PCR was used to confirm the kpc gene in MHT-positive P. aeruginosa with 5 different specific primers (Table 1 [Tab. 1], Figure 1 [Fig. 1]). PCR program was performed as follows. Initial denaturation for each of the genes was performed at 95°C for 5 min and thereafter 30 cycles with denaturation at 95°C for 1 min. The annealing temperatures consisted of blaKPC 64°C, blaKPCA 56°C, blaKPCB 56°C, blaKPCc 60°C and blaKPCD 55°C , the annealing time was 1 min. The extension time was 1 min at 72°C. The final extension for all genes was done at 72°C for 5 min.
Table 1: Five different primer sequences used in this study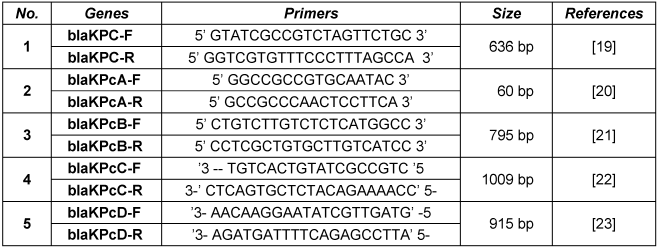
Figure 1: PCR amplification fragments for detection of the kpc gene among Pseudomonas aeruginosa isolates 
M: 1kb DNA size marker; lane 1–3 and 5–7: punctuation here is confusing negative strains; lane 4: negative control; lane 8 and 10: positive strains.
Results
In this study, 241 strains were identified and confirmed as P. aeruginosa. One hundred eighty-six strains were resistant to all tested carbapenems (Imipenem, Meropenem and Ertapenem). Seventy-five strains had MHT-positive test results. A synergism effect between Meropenem and APBA was observed in 11 P. aeruginosa strains with positive MHT test results. On the other hand, only 3 strains had synergism with APBA alone and the remaining 8 strains had synergism with Oxacillin simultaneously. Further, PCR with different specific primers showed specific bands after gel electrophoresis in only two MHT-positive strains with primer No. 1. The proportion of positive results from molecular testing of carbapenem-resistant P. aeruginosa is 0.01 (1.07%). One of two strains had a synergistic effect with APBA.
Discussion
Resistance to carbapenems in P. aeruginosa is a growing problem worldwide [1], [2], [3]. Resistance to carbapenems associated with KPC production is an alarming problem for health care systems [1], because the kpc gene can transfer between P. aeruginosa strains or even from P. aeruginosa to enterobacteria [4], [5]. In addition, KPC-producing carbapenem-resistant P. aeruginosa strains have potential resistance to all betalactam antibiotics except Azthronam [5], [6]. This can cause complications in the treatment of infections related to KPC-producing bacteria.
Our findings showed that MHT had an excellent sensitivity (100%) but low specificity (2.6%) for the detection of KPC among Iranian bacterial specimens. We suggest that MHT can be used as a primary screening test, since only two out of 75 MHT-positive strains were confirmed by PCR. In Argentina, sensitivity and specificity of MHT for carbapenemase-producing P. aeruginosa were reported to be 78% and 57%, respectively [14], [15]. However, the use of inhibitor boronic acid led to a significant increase of 97% in sensitivity and specificity [14]. Despite high sensitivity, MHT can have variable specificity due to different incidences of carbapenemase in different geographical areas (57%–≥90%) [12], [14], [15]. For instance, in Colombia in 2007, three KPC-producing P. aeruginosa strains were identified [16], and in 2009, one KPC-producing P. aeruginosa strains was isolated [17]. A study conducted in the USA in 2009 reported one KPC-producing P. putida strain and one E. cloacae strain from one patient, where MHT was positive for both [18]. Additionally, in 2010, one KPC-producing P. aeruginosa strain was isolated in the USA [2]. Despite global emergence of KPC-producing strains of P. aeruginosa, it still uncommon and is thought to be sporadic (yet its rapid detection is necessary) [2], [16], [17], [18]. In our study, two KPC-producing P. aeruginosa strains were identified, which is the first report from Iran to the best of our knowledge. On the other hand, the results of this study confirmed that only one of three strains with positive result in combination disk method (meropenem plus APBA) confirmed by PCR as a KPC producer.
These findings indicate that the sensitivity and specificity of inhibitory test (use of boronic acid) could not be explained by our results. This is despite the fact that false positive results were eliminated using Oxacillin plus Meropenem in our methodology. Thus, our findings may suggest that MHT, despite its excellent sensitivity, has variable specificity dependent of species of isolated bacteria and also depending on different geographical areas. Also, the use of a KPC inhibitor such as boronic acid may not lead to a reasonably high sensitivity and specificity among Iranian bacterial specimens. Therefore, PCR should be considered as the gold standard for the detection of KPC-producing P. aeruginosa for the time being.
Notes
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by a grant (M/T 91-01-134-17129) from Tehran University of Medical Sciences, Iran.
References
[1] Vahdani M, Azimi L, Asghari B, Bazmi F, Rastegar Lari A. Phenotypic screening of extended-spectrum ß-lactamase and metallo-ß-lactamase in multidrug-resistant Pseudomonas aeruginosa from infected burns. Ann Burns Fire Disasters. 2012 Jun 30;25(2):78-81. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3506211/[2] Poirel L, Nordmann P, Lagrutta E, Cleary T, Munoz-Price LS. Emergence of KPC-producing Pseudomonas aeruginosa in the United States. Antimicrob Agents Chemother. 2010 Jul;54(7):3072. DOI: 10.1128/AAC.00513-10
[3] Robledo IE, Aquino EE, Vázquez GJ. Detection of the KPC gene in Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii during a PCR-based nosocomial surveillance study in Puerto Rico. Antimicrob Agents Chemother. 2011 Jun;55(6):2968-70. DOI: 10.1128/AAC.01633-10
[4] Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns. 2013 Feb;39(1):174-6. DOI: 10.1016/j.burns.2012.02.025
[5] Sidjabat HE, Silveira FP, Potoski BA, Abu-Elmagd KM, Adams-Haduch JM, Paterson DL, Doi Y. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin Infect Dis. 2009 Dec;49(11):1736-8. DOI: 10.1086/648077
[6] Azimi L, Rastegar Lari A, Alaghehbandan R, Alinejad F, Mohammadpoor M, Rahbar M. KPC-producer gram negative bacteria among burned infants in Motahari Hospital, Tehran: first report from Iran. Ann Burns Fire Disasters. 2012 Jun 30;25(2):74-7. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3506210/
[7] Limbago BM, Rasheed JK, Anderson KF, Zhu W, Kitchel B, Watz N, Munro S, Gans H, Banaei N, Kallen AJ. IMP-producing carbapenem-resistant Klebsiella pneumoniae in the United States. J Clin Microbiol. 2011 Dec;49(12):4239-45. DOI: 10.1128/JCM.05297-11
[8] Roh KH, Lee CK, Sohn JW, Song W, Yong D, Lee K. Isolation of a Klebsiella pneumoniae isolate of sequence type 258 producing KPC-2 carbapenemase in Korea. Korean J Lab Med. 2011 Oct;31(4):298-301. DOI: 10.3343/kjlm.2011.31.4.298
[9] Carbonne A, Thiolet JM, Fournier S, Fortineau N, Kassis-Chikhani N, Boytchev I, Aggoune M, Seguier JC, Senechal H, Tavolacci MP, Coignard B, Astagneau P, Jarlier V. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Euro Surveill. 2010 Dec 2;15(48). pii: 19734. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19734
[10] Public Health Agency of Canada. Guidance: Infection Prevention and Control Measures for Healthcare Workers in All Healthcare Setting. [modified 2012-04-03]. Available from: http://www.phac-aspc.gc.ca/nois-sinp/guide/ipcm-mpci/ipcm-mpci-eng.php
[11] Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. M100-S19. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
[12] Girlich D, Poirel L, Nordmann P. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol. 2012 Feb;50(2):477-9. DOI: 10.1128/JCM.05247-11
[13] De Vos D, Lim A Jr,Pirnay JP, Struelens M, Vandenvelde C, Duinslaeger L, Vanderkelen A, Cornelis P. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J Clin Microbiol. 1997 Jun;35(6):1295-9. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC229737/
[14] Pasteran F, Veliz O, Faccone D, Guerriero L, Rapoport M, Mendez T, Corso A. A simple test for the detection of KPC and metallo-ß-lactamase carbapenemase-producing Pseudomonas aeruginosa isolates with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect. 2011 Sep;17(9):1438-41. DOI: 10.1111/j.1469-0691.2011.03585.x
[15] Pasteran F, Veliz O, Rapoport M, Guerriero L, Corso A. Sensitive and specific modified Hodge test for KPC and metallo-beta- lactamase detection in Pseudomonas aeruginosa by use of a novel indicator strain, Klebsiella pneumoniae ATCC 700603. J Clin Microbiol. 2011 Dec;49(12):4301-3. DOI: 10.1128/JCM.05602-11
[16] Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, Quinn JP; the Colombian Nosocomial Resistance Study Group. First identification of Pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing beta-lactamase. Antimicrob Agents Chemother. 2007 Apr;51(4):1553-5. DOI: 10.1128/AAC.01405-06
[17] Akpaka PE, Swanston WH, Ihemere HN, Correa A, Torres JA, Tafur JD, Montealegre MC, Quinn JP, Villegas MV. Emergence of KPC-producing Pseudomonas aeruginosa in Trinidad and Tobago. J Clin Microbiol. 2009 Aug;47(8):2670-1. DOI: 10.1128/JCM.00362-09
[18] Bennett JW, Herrera ML, Lewis JS 2nd,Wickes BW, Jorgensen JH. KPC-2-producing Enterobacter cloacae and pseudomonas putida coinfection in a liver transplant recipient. Antimicrob Agents Chemother. 2009 Jan;53(1):292-4. DOI: 10.1128/AAC.00931-08
[19] Wolter DJ, Khalaf N, Robledo IE, Vázquez GJ, Santé MI, Aquino EE, Goering RV, Hanson ND. Surveillance of carbapenem-resistant Pseudomonas aeruginosa isolates from Puerto Rican Medical Center Hospitals: dissemination of KPC and IMP-18 beta-lactamases. Antimicrob Agents Chemother. 2009 Apr;53(4):1660-4. DOI: 10.1128/AAC.01172-08
[20] Kitchel B, Rasheed JK, Endimiani A, Hujer AM, Anderson KF, Bonomo RA, Patel JB. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2010 Oct;54(10):4201-7. DOI: 10.1128/AAC.00008-10
[21] Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob Agents Chemother. 2008 Apr;52(4):1257-63. DOI: 10.1128/AAC.01451-07
[22] Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001 Apr;45(4):1151-61. DOI: 10.1128/AAC.45.4.1151-1161.2001




