[Zur Qualität bakteriologisch-infektionsserologischer Verfahren in Deutschland: Auswertung der infektionsserologischen Ringversuche 2016]
Renata Smit 1,2Klaus-Peter Hunfeld 1,2,3
1 Institute for Laboratory Medicine, Microbiology and Infection Control, Northwest Medical Centre, Academic Teaching Hospital, Medical Faculty, Goethe University, Frankfurt am Main, Germany
2 INSTAND e.V., Düsseldorf, Germany
3 German Society for Hygiene and Microbiology, Safety of Quality commission, Hannover, Germany
Zusammenfassung
Serologische Tests zum Nachweis von erregerspezifischen Antikörpern und Antigenen sind nach wie vor ein diagnostischer Goldstandard in der Infektionsserologie. Daher sind standardisierte und genaue Testverfahren äußerst wichtig. Sie können auch dazu beitragen, die Qualität der Behandlung und Patientenversorgung zu verbessern. In der vorliegenden Arbeit werden die Ergebnisse der bakteriologischen Infektionsserologie in Ringversuchen für das Jahr 2016 in Deutschland zusammengefasst und diskutiert.
Schlüsselwörter
externe Qualitätssicherung, EQA, Ringversuche, bakteriologische Infektionsserologie, Deutschland
1 Introduction
Serology is currently making a significant contribution to the etiology, prognosis and treatment success of many infectious diseases. It also serves as an efficient way to confirm past infections and determine an individual’s vaccination/protection status. Therefore, serological testing that detects pathogen-specific antibodies and antigens plays an important diagnostic role in infectious serology [1].
The accuracy of the assays is essential since there are many test systems on the market and an insufficient amount of standardization [2]. External quality assurance (EQA) is a well-established method that enables inter-laboratory comparisons by way of proficiency testing trials in order to assess the quality of results by an external agency [3]. In Germany, EQA is conducted by the Society for Promoting Quality Assurance in Medical Laboratories e.V. (INSTAND e.V., Düsseldorf) on behalf of the German Medical Association and in collaboration with other partners (i.e. German Society for Hygiene and Microbiology, DGHM) [1]. EQA forms the basis for the development of strategies to improve diagnostic processes and for the continuous improvement of analytical results [1]. It can also help to improve the quality of treatment and patient care [4]. This paper summarizes and discusses the results of the infectious serology as part of proficiency testing trials conducted in 2016. The findings can help to improve the diagnosis of individual constellations and to optimize the test systems used. This paper deals with a standardized form.
2 Methods
2.1 Participants
In 2016, 12,506 laboratories participated in two proficiency testing trials. 10,674 participants were from Germany and 1,340 were from other European countries. One trial was carried out in May and the other in November 2016 (Table 1 [Tab. 1]).
Table 1: Study groups and the number of participants included in two proficiency testing trials in Germany, 2016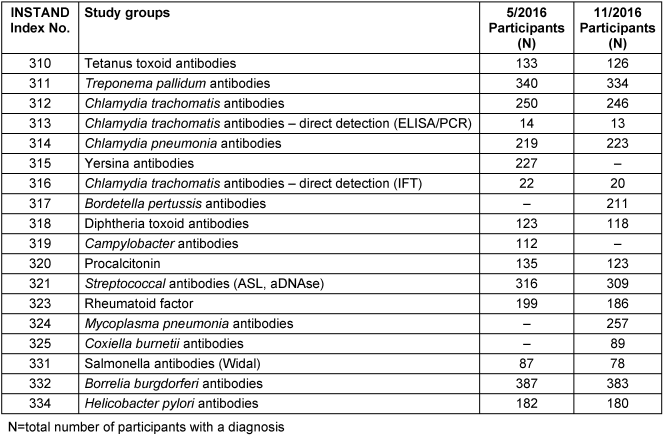
2.2 Sample collection and EQA progress
The control samples (31, 32, 61 and 62) for each study group (310–334) originated from the whole blood of healthy donors or from blood donors with a positive history of infection, and were prepared according to standard operating procedures [5]. Samples 31 and 32 were sent to participating laboratories in May 2016, while samples 61 and 62 were sent in November 2016 [5]. The control samples of Yersinia, Bordetella pertussis, Campylobacter, Mycoplasma pneumonia and Coxiella burnetii were only analyzed once that year [6], [7].
Participants performed serological analyses on samples from each study group using routine procedures and documented the results and test system used [5]. All results were recorded on the computer and statistically evaluated. When the results were in line with certified reference standards, a certificate was issued by INSTAND e.V. [4].
2.3 Target values
The reference measurement procedure, which follows the guideline of the German Medical Association (RiLiBÄK), enables target values to be determined with the highest level of accuracy and precision. When a uniform target value cannot be determined for the quantitative test results, the robust mean of all participants is established as the target value. In terms of the qualitative test results, either the mode of the results of the reference laboratories, or the mode of the results of the participants is set as the target value [6], [7]. Qualitative test results are expressed as positive, negative or borderline, while semi-quantitative test results are provided in the form of titers, cut-off indices or U/ml. A specific assay or test method should be evaluated in a collective of more than eight participants. If the number of participants is less than eight, this could lead to statistically invalid assessments. Therefore, certificates for the test method are only issued for a collective of eight or more participants [6], [7].
3 Results
3.1 Tetanus toxoid (310)
3.1.1 Sample information
All samples (31, 32, 61 and 62) originated from clinically healthy blood donors.
3.1.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the robust mean of all participants was established as the target value. The corresponding target values, target ranges, test results and pass rates are listed in Table 2 [Tab. 2].
Table 2: Tetanus toxoid detection during the proficiency testing trials 2016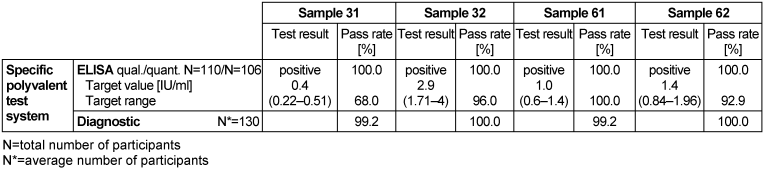
3.1.3 Overall diagnostic interpretation and commentary on the test results
ELISA was used to quantitatively determine antibodies against tetanus toxin and toxoid (TT) in the serum of clinically healthy donors. Antibody detection has no diagnostic relevance in determining a tetanus infection, however, it can be used to assess an individual’s immune or vaccination status for tetanus toxoid [1]. Results of this trial show that samples 32, 61 and 62 had tetanus toxoid antibody titers ranging from 1 to 2.9 IU/ml, suggesting protective immunity against tetanus toxoid. However, the level of antibody titers in samples 32 and 62 indicated that a booster vaccination will be needed in 5 to 10 years for long-term protection, while in sample 61 a booster vaccination in 2 to 5 years will be needed to provide long-term protection. The level of antibodies in sample 31 was 0.4 IU/ml, suggesting that there was protective immunity; however, a booster vaccination will provide long-term immunity. It is important to note that vaccinations should primarily be in accordance with the recommendations of the German Standing Committee on Vaccination (STIKO) and not just based on measured antibody levels [1]. Pass rates for ELISA tests were 100% for the qualitative results and 99.2–100% for the quantitative results. The pass rate for the overall clinical diagnostic evaluation was between 99.2 and 100%.
3.2 Treponema pallidum antibodies (311)
3.2.1 Sample information
Samples 32 and 62 originated from healthy blood donors without any clinical evidence of syphilis. Sample 31 was taken from a clinically asymptomatic blood donor before a scheduled donation. The donor could not recall any previous infection or treatment. Sample 61 was donated by a blood donor treated for a syphilis infection several years ago.
3.2.2 Determination of the target values
The target value for the qualitative test results was the mode of the results of the reference laboratories, while in the case of the semi-quantitative test results, the results of the reference laboratories were established as the target value. The corresponding target values, target ranges, test results and pass rates are presented in Table 3 [Tab. 3].
Table 3: Treponema pallidum antibody detection during the proficiency testing trials 2016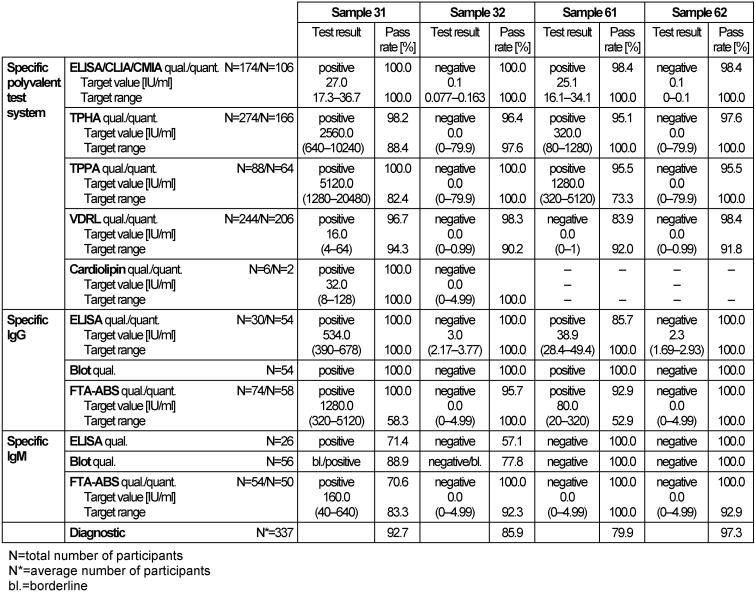
3.2.3 Overall diagnostic interpretation and commentary on the test results
Samples 32 and 62 showed no clinical or serological evidence of a syphilis infection. However, false reactive test results were reported for sample 32, mainly for IgM immunoblot, although the sample tested negative by the IgM-FTA-ABS test. The positive serological findings of sample 31 (target value (mode): TPPA: 5120, VDRL: positive, 16, IgM-FTA-ABS: positive, 160, EIA and immunoblot for IgG: positive and for IgM: borderline or positive), indicated a latent or possibly active infection. The overall pass rate of all test methods for sample 31 was 92.7%. The positive sample 61 (target value: TPPA: 1280, polyval. ELISA, IgG-ELISA: positive, VDRL: negative/borderline, FTA-ABS-IgG: 80, FTA-ABS-IgM and IgM-ELISA: negative) achieved an overall pass rate of 79.9%, indicating unsatisfactory test sensitivity. Findings in both samples 31 and 61 indicated that treatment was needed. The distribution of the immunoblot bands, as reported for the positive samples 31 (Figure 1 [Fig. 1], Figure 2 [Fig. 2]) and 61 (Figure 3 [Fig. 3]), are shown below [6], [7]. The overall diagnostic evaluation of the negative samples 32 and 62 showed pass rates between 85.9 and 97.3%, whereas those of the positive samples 31 and 61 were between 79.9 and 92.7%.
Figure 1: The distribution of the immunoblot bands reported for the positive sample 31 [7], [8]; recovery rate (%) of the submitted IgG immunoblot bands for sample 311/31 (May 2016); participants N=149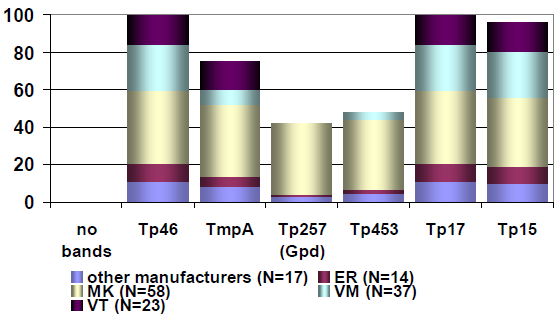
Figure 2: The distribution of the immunoblot bands reported for the positive sample 31 [7], [8]; recovery rate (%) of the submitted IgM immunoblot bands for sample 311/31 (May 2016); participants N=158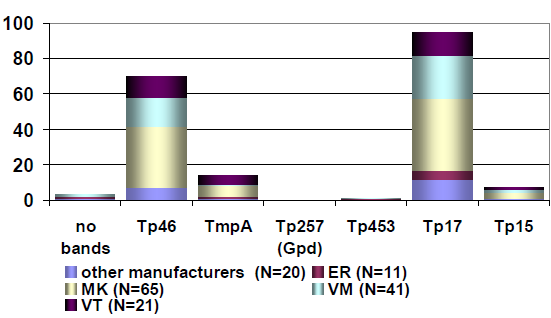
Figure 3: The distribution of immunoblot bands reported for the positive sample 61 [7], [8]; recovery rate (%) of the submitted IgG immunoblot bands for sample 311/61 (Nov. 2016); participants N=151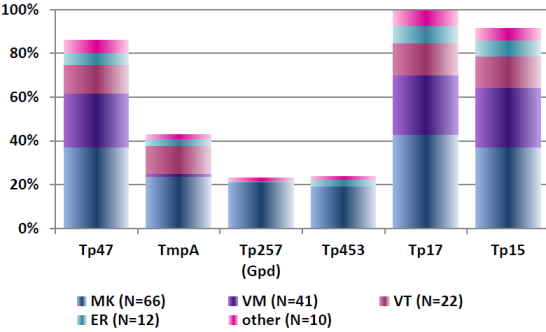
3.3 Chlamydia trachomatis antibodies (312)
3.3.1 Sample information
All samples (31, 32, 61 and 62) originated from clinically healthy blood donors.
3.3.2 Determination of the target values
The target value for the qualitative test results was the mode of the results of the reference laboratories; in the case of the semi-quantitative results, the results of the reference laboratories were set as the target value. The corresponding target values, target ranges, test results and pass rates are shown in Table 4 [Tab. 4].
Table 4: Chlamydia trachomatis antibody detection during the proficiency testing trials 2016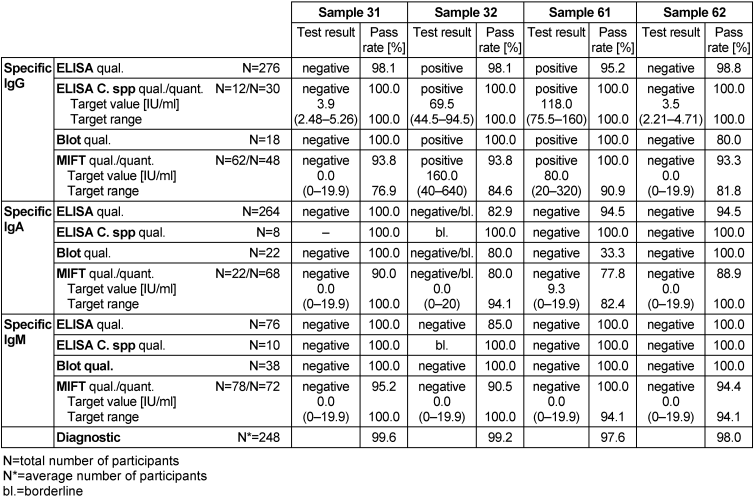
3.3.3 Overall diagnostic interpretation and commentary on the test results
Samples 31 and 62 showed no serological evidence of a C. trachomatis infection. IgG antibodies were detected in sample 32, combined with negative test results for IgM antibodies and borderline results for IgA antibodies. IgG antibodies were detected in sample 61, combined with negative results for both IgM and IgA antibodies. Consequently, the test results for both samples 32 and 62 indicated an infection with C. trachomatis. In sample 61, the pass rate was between 33.3 and 100% for all tests, with the lowest pass rate (33.3%) for the qualitative detection of IgA antibodies by immunoblot. The overall diagnostic evaluation of the negative samples was 97.6–99.2% and thus in the range of previous years, while positive samples were more or less in the same range (98–99.6%).
3.4 Chlamydia trachomatis antibodies – direct detection by ELISA/PCR (313)
3.4.1 Sample information
All samples (31, 32, 61 and 62) originated from clinically healthy blood donors.
3.4.2 Determination of the target values
The mode of the results of all participants was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the mode of the results of the reference laboratories was established as the target value. The corresponding target values, target ranges, test results and pass rates are presented in Table 5 [Tab. 5].
Table 5: Chlamydia trachomatis antibody detection during the proficiency testing trials 2016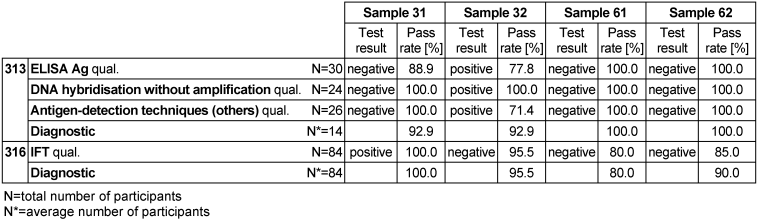
3.4.3 Overall diagnostic interpretation and commentary on the test results
Samples 31, 61 and 62 showed no clinical or serological evidence of C. trachomatis, while sample 32 tested positive for the pathogen, indicating a C. trachomatis infection. The overall diagnostic evaluation of the negative samples 31, 61 and 62 was between 92.9–100% and thus in line with previous years, while the positive sample 32 was 92.9%.
3.5 Chlamydia pneumonia antibodies (314)
3.5.1 Sample information
All samples (31, 32, 61 and 62) were taken from clinically healthy blood donors.
3.5.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results; in the case of the semi-quantitative test results, the results of the reference laboratories were specified as the target value. The corresponding target values, target ranges, test results and pass rates are shown in Table 6 [Tab. 6].
Table 6: Chlamydia pneumonia antibody detection during the proficiency testing trials 2016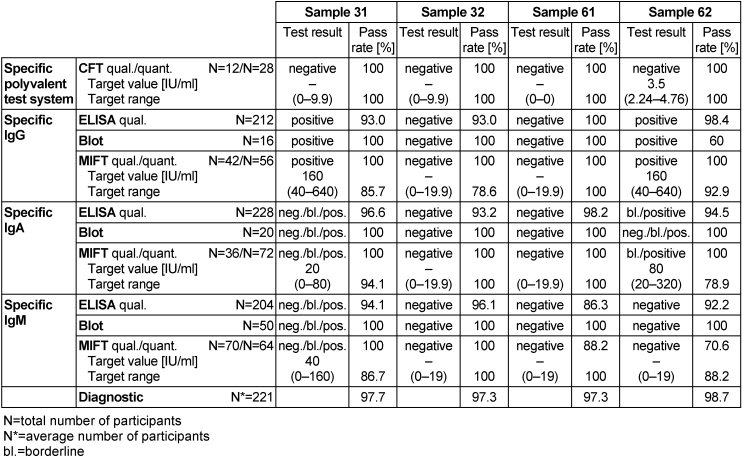
3.5.3 Overall diagnostic interpretation and commentary on the test results
No evidence of an infection with C. pneumonia was detected in samples 32 and 61, while samples 31 and 62 tested positive for the pathogen. In sample 31, IgG antibodies and borderline results for IgA and IgM antibodies were detected, while in sample 62, IgG antibodies were detected along with borderline results for IgA antibodies. Test results in both samples indicated an infection with C. pneumonia. All four samples had qualitative results of 86.3–100% for all ELISA tests, while all MIFT tests were in a range of 70.6–100% for qualitative results and 78.6–100% for quantitative results. The qualitative results of all immunoblot tests for all samples except for sample 62 were 100%. In sample 62, immunoblot had a pass rate of 60% for detecting IgG antibodies. The overall diagnostic evaluation of the negative samples 32 and 61 was 97.3%, while for the positive samples 31 and 62 it was 97.7–98.7%.
3.6 Yersinia antibodies (315)
3.6.1 Sample information
Sample 31 was obtained from a patient with reactive arthritis. Sample 32 was collected from a clinically asymptomatic seronegative blood donor.
3.6.2 Determination of the target values
The mode of the results of all participants was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the results of the reference laboratories were set as the target value. The corresponding target values, target ranges, test results and pass rates are listed in Table 7 [Tab. 7].
Table 7: Yersinia antibody detection during the proficiency testing trials 2016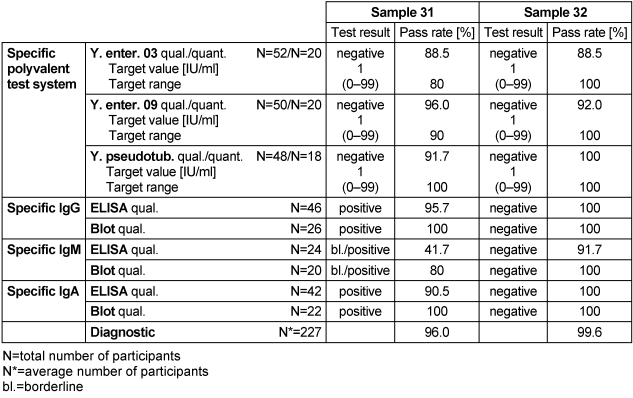
3.6.3 Overall diagnostic interpretation and commentary on the test results
No evidence of an infection with Yersinia was detected in sample 32, while sample 31 tested positive for the pathogen. Sample 31 had negative Widal test results, but clear reactive results for the immunoglobulin classes IgG and IgA by ELISA and immunoblot. Borderline results for IgM antibodies were detected by ELISA and immunoblot. The constellation of findings in sample 31 was consistent with both a previous Yersinia infection (this probably occurred a long time ago because the Widal test was negative) and with a Yersinia-associated secondary disease [6], [7]. The overall diagnostic evaluation of the negative sample 32 was 99.6%, while it was 96% for the positive sample 31.
3.7 Chlamydia trachomatis antibodies – direct detection by IFT (316)
3.7.1 Sample information
All samples (31, 32, 61 and 62) were taken from clinically healthy blood donors.
3.7.2 Determination of the target values
The mode of the results of all participants was established as the target value for the qualitative test results. The corresponding target values, target ranges, test results and pass rates are listed in Table 5 [Tab. 5].
3.7.3 Overall diagnostic interpretation and commentary on the test results
Samples 32, 61 and 62 showed no serological evidence of C. trachomatis, while sample 31 tested positive for the pathogen, indicating an infection with C. trachomatis. The overall diagnostic evaluation of the negative samples 32, 61 and 62 was between 80–95.5% and thus in the line with previous years, while the pass rate of positive sample 31 was 100%.
3.8 Bordetella pertussis antibodies (317)
3.8.1 Sample information
Sample 61 was donated by a non-immunized blood donor without evidence of any respiratory infections in his recent medical history. Sample 62 originated from a healthy vaccinated blood donor without any respiratory infections in his recent medical history.
3.8.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the robust mean of all participants was stipulated as the target value. The corresponding target values, target ranges, test results and pass rates are listed in Table 8 [Tab. 8].
Table 8: Bordetella pertussis antibody detection during the proficiency testing trials 2016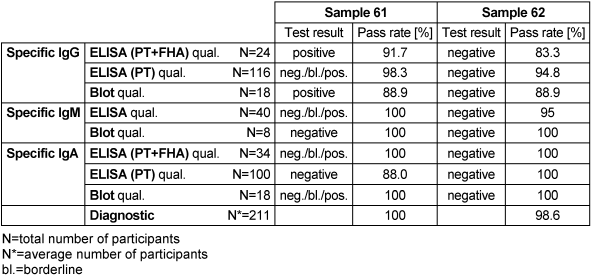
3.8.3 Overall diagnostic interpretation and commentary on the test results
Sample 62 tested negative for specific antibodies against B. pertussis; therefore, no evidence of infection was detected. Sample 61 tested positive for the pathogen. This sample exhibited an interesting constellation of findings in different assay systems and yielded weakly reactive results for IgG as well as for IgM and IgA. PT-based tests resulted in borderline or positive results (IgG-ELISA, IgG-immunoblot). The quantitative PT-IgG-ELISA result was 44.6 IU/ml, indicating a doubtful result or the possibility of a past infection according to the guidelines [6], [7]. Due to the overall variability of the findings, however, the survey was generous and the clinical commentary as to a recent infection was accepted. The overall diagnostic evaluation of the negative sample 62 was 98.6%, while for the positive sample 61 it was 100%.
3.9 Diphtheria toxoid antibodies (318)
3.9.1 Sample information
Samples 31 and 32 were donated by healthy pre-immunized blood donors, while samples 61 and 62 originated from clinically healthy blood donors.
3.9.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the robust mean of all participants was stipulated as the target value. The corresponding target values, target ranges, test results and pass rates are shown in Table 9 [Tab. 9].
Table 9: Diphtheria toxoid antibody detection during the proficiency testing trials 2016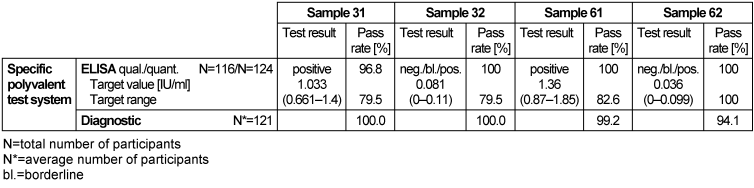
3.9.3 Overall diagnostic interpretation and commentary on the test results
Antibodies against the diphtheria toxin and toxoid (DT) can be quantitatively detected in serum.
Antibody detection is not suitable for identifying an acute case of diphtheria; it can only be used to assess the immune and vaccination status of diphtheria [1].
The titer level (1.033 IU/ml) of sample 31 indicated protective immunity, however, a booster vaccination would provide long-term immunity. The high titer level of 1.36 IU/ml in sample 61 indicated that there was active protective immunity against tetanus, however a booster vaccination in 5 to 10 years would be needed to provide long-term protection. Low titer levels of 0.081 IU/ml and 0.036 IU/ml in samples 32 and 62 respectively suggested that there was no protective immunity; therefore, a booster vaccination was recommended. Vaccination recommendations should primarily be made following STIKO recommendations and based on measured antibody levels [1]. ELISA tests had qualitative results of 96.8–100% and quantitative results of 79.5–100%. The overall diagnostic evaluation was in the range of 94.1–100%.
3.10 Campylobacter antibodies (319)
3.10.1 Sample information
Samples 31 and 32 originated from clinically healthy blood donors.
3.10.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the robust mean of all participants was stipulated as the target value. The corresponding target values, target ranges, test results and pass rates are listed in Table 10 [Tab. 10].
Table 10: Campylobacter antibody detection during the proficiency testing trials 2016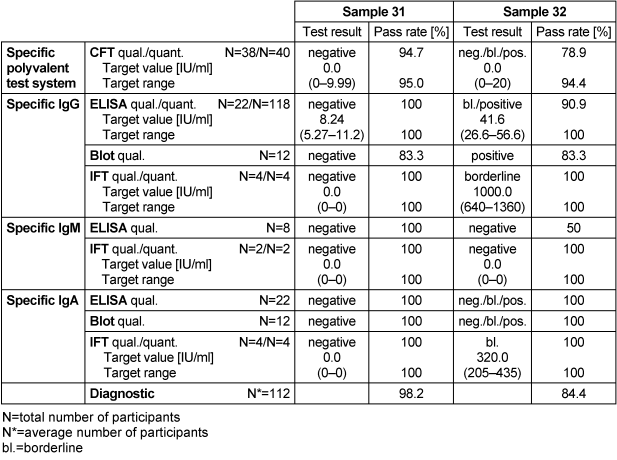
3.10.3 Overall diagnostic interpretation and commentary on the test results
Specific IgG, IgM and IgA antibodies were detected using the commercially available ELISA, immunoblot and IFT tests. No evidence of an infection with C. jejuni was detected in sample 31, while sample 32 had borderline results for IgG and IgA antibodies against the tested pathogen, indicating a Campylobacter infection. In sample 31, qualitative and quantitative results for ELISA were 100% for IgG, IgM and IgA antibodies against C. jejuni, while in sample 32 they were in the range of 50–100%. Sample 32 had a low qualitative result of 50% for IgM antibodies using the ELISA method. The overall diagnostic evaluation of the negative sample 31 was 98.2%, while it was 84.4% for the positive sample 32.
3.11 Procalcitonin (320)
3.11.1 Sample information
Samples 31, 32 and 61 were donated by a healthy blood donor without signs of infection. Sample 62 was pooled from backup samples of septic patients.
3.11.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while the robust mean of all participants was stipulated as the target value for the semi-quantitative test results. The corresponding target values, target ranges, test results and pass rates are presented in Table 11 [Tab. 11].
Table 11: Procalcitonin detection during the proficiency testing trials 2016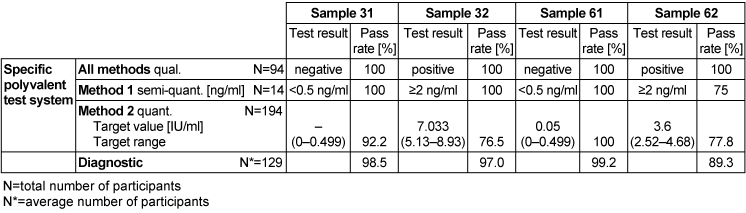
3.11.3 Overall diagnostic interpretation and commentary on the test results
Findings for samples 31 and 61 indicated that a local bacterial infection was possible in both samples, while findings for samples 32 and 62 indicated the likelihood of a systemic infection (sepsis). Semi-quantitative results of method 1 for samples 31 and 61 were <0.5 ng/ml with a pass rate of 100%, while samples 32 and 62, at ≥2 ng/ml, had pass rates of 100% and 75% respectively.
In samples where a local bacterial infection was likely, the overall diagnostic evaluation was between 98.5 and 99.2%, while in samples where sepsis was possible, it was between 89.3 and 97%.
3.12 Streptococci antibodies (321)
3.12.1 Sample information
All samples (31, 32, 61 and 62) were donated by a healthy blood donor.
3.12.2 Determination of the target values
Different testing methods were used to determine the streptococcal antibodies (DNSAse and the anti-Streptolysin-O) against streptococcal antigens. For the qualitative test results, the mode of the results of the reference laboratories was set as the target value and it depended on the method used. In the case of the semi-quantitative test results, the robust mean of all participants was specified as the target value. The range of acceptance for the semi-quantitative results was ±25%. The corresponding target values, target ranges, test results and pass rates are displayed in Table 12 [Tab. 12].
Table 12: Streptococci antibody detection during the proficiency testing trials 2016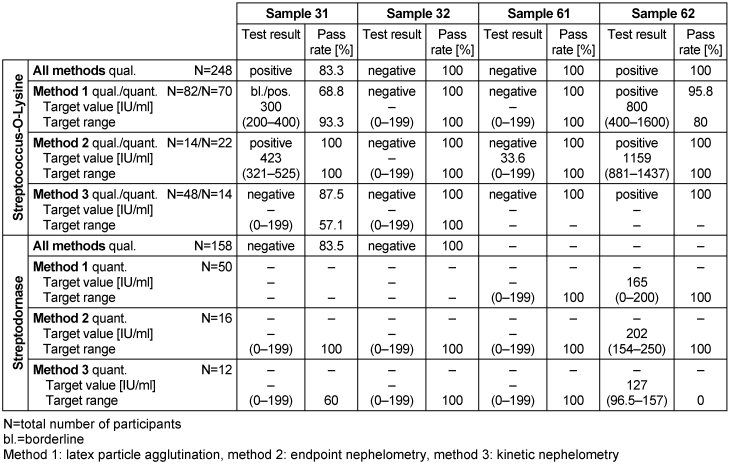
3.12.3 Overall diagnostic interpretation and commentary on the test results
In the qualitative evaluation, titers of streptococcal antibodies above the cut-off value (200 IU/ml) indicated an infection with Streptococcus. Titers between 200 and 400 indicated a past or recent infection [5]. A much higher titer occurs when there is severe infection or an acute secondary disease. The pass rate of Streptococcus-O-lysine antibody detection was between 57.3 and 100%, and the pass rate of streptodornase detection was between 60 and 100%.
3.13 Rheumatoid factor (323)
3.13.1 Sample information
All samples (31, 32, 61 and 62) were taken from clinically healthy blood donors.
3.13.2 Determination of the target values
The mode of the results of reference laboratories was set as the target value for the qualitative test results, while the robust mean of all participants was stipulated as the target value for the semi-quantitative test results. The corresponding target values, target ranges, test results and pass rates are indicated in Table 13 [Tab. 13].
Table 13: Rheumatoid factor detection during the proficiency testing trials 2016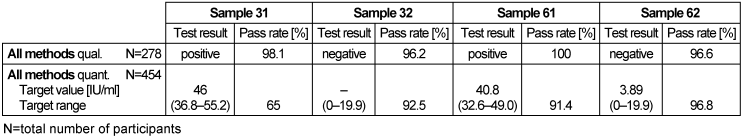
3.13.3 Overall diagnostic interpretation and commentary on the test results
The quantitative and qualitative test results for all methods were between 65–100%, with the worst qualitative results being 65% for sample 31.
3.14 Mycoplasma pneumonia antibodies (324)
3.14.1 Sample information
Sample 61 originated from a patient with several known respiratory infections in his recent medical history. Sample 62 was donated by a healthy blood donor in the summer months.
3.14.2 Determination of the target values
The mode of the results of all participants was set as the target value for the qualitative test results, while for the semi-quantitative test results, the robust mean of all participants was stipulated as the target value. The corresponding target values, target ranges, test results and pass rates are presented in Table 14 [Tab. 14].
Table 14: Mycoplasma pneumonia antibody detection during the proficiency testing trials 2016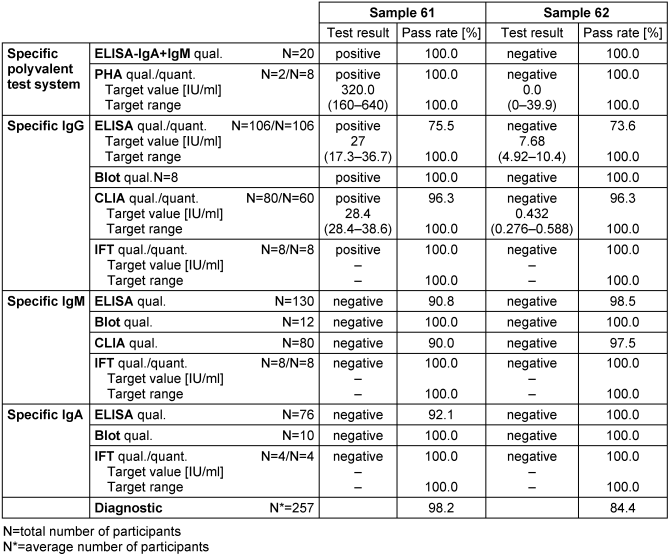
3.14.3 Overall diagnostic interpretation and commentary on the test results
No evidence of an infection with M. pneumonia was detected in sample 62, while in sample 61 both the reference laboratories and most participants detected a pronounced IgG seroreactivity combined with negative test results for specific IgM and IgA antibodies. Consequently, the test results pointed to a past infection with M. pneumonia. The overall diagnostic evaluation of the negative sample 62 was 84.4%, while for sample 61 it was 98.2%.
3.15 Coxiella burnetii antibodies (325)
3.15.1 Sample information
Sample 61 was donated by a patient shortly after an acute infection with C. burnetii. Sample 62 was donated by a healthy blood donor without evidence of a recent infection.
3.15.2 Determination of the target values
The mode of the results of all participants was set as the target value for the qualitative test results, while the robust mean of all participants was stipulated as the target value of the semi-quantitative test results. The corresponding target values, target ranges, test results and pass rates are shown in Table 15 [Tab. 15].
Table 15: Coxiella burnetii antibody detection during the proficiency testing trials 2016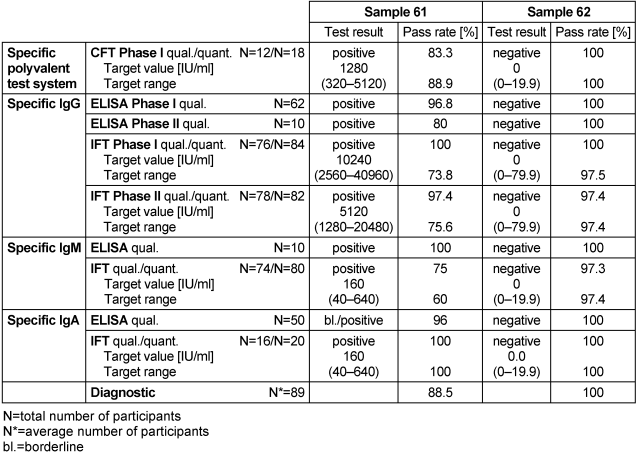
3.15.3 Overall diagnostic interpretation and commentary on the test results
Sample 62 tested negative for C. burnetii, while sample 61 tested positive. The positive sample 61 had CFT titers of 1280 for phase I and 640 for phase II (median), IgG phase I – IFT titers of 10240 and IgG phase II – IFT titers of 5120 (median), positive IgM test results as well as weakly reactive IgA test results. This indicated an acute infection with C. burnetii [6], [7].
The overall diagnostic evaluation of the negative sample 62 was 100% and thus in line with previous years, while the pass rate of positive sample 61 was 88.5%.
3.16 Salmonella antibodies (331)
3.16.1 Sample information
All samples (31, 32, 61 and 62) originated from clinically healthy blood donors.
3.16.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while in the case of the semi-quantitative results, the results of the reference laboratories were set as the target value. The corresponding target values, target ranges, test results and pass rates are listed in Table 16 [Tab. 16].
Table 16: Salmonella antibody detection during the proficiency testing trials 2016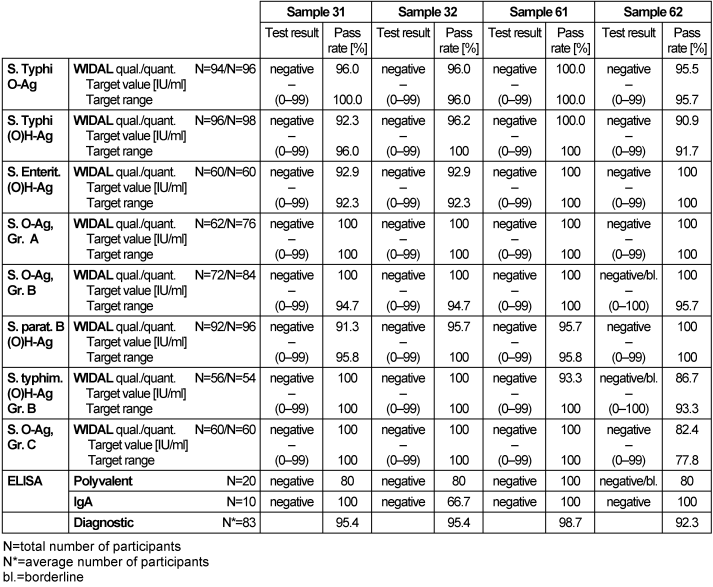
3.16.3 Overall diagnostic interpretation and commentary on the test results
No evidence of a Salmonella infection was detected in samples 31, 32 and 61. In sample 62 borderline results for Salmonella Ag. Gr A, Salmonella typhim. and (O)H-Ag Gr. B antibodies against the tested pathogen were detected using WIDAL tests. The borderline results were also detected for the specific polyvalent ELISA. These results pointed to a possible infection or no infection. The pass rates for the Widal test system were between 91.5 and 100%, while for ELISA they were between 80 and 100%. The overall diagnostic pass rates were between 92.3 and 100%, showing better results than in previous years.
3.17 Borrelia burgdorferi antibodies (332)
3.17.1 Sample information
Samples 31, 61 and 62 originated from a healthy blood donor without evidence of a tick bite or clinical Lyme borreliosis in his medical history. Sample 32 was donated by a patient with recent neuroborreliosis (CSF pleocytosis, positive borrelia-specific antibody index).
3.17.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results, while in the case of the semi-quantitative test results, the mode of the results of all participants was set as the target value. The corresponding target values, target ranges, evaluation and pass rates are listed in Table 17 [Tab. 17].
Table 17: Borrelia burgdorferi antibody detection during the proficiency testing trials 2016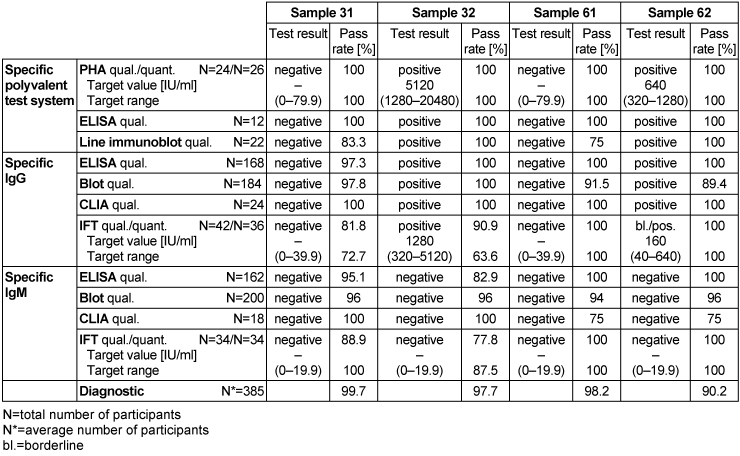
3.17.3 Overall diagnostic interpretation and commentary on the test results
No evidence of an infection with B. burgdorferi was detected in samples 31 and 61, while pathogens were detected in samples 32 and 62, indicating an infection with B. burgdorferi.
Sample 62 showed isolated reactivity for specific IgG antibodies against B. burgdorferi in ELISA, immunoblot and CLIA, with borderline test results in IFT and negative test results for specific IgM antibodies in all tests systems. Sample 32 showed positive test results for specific IgG antibodies and negative test results for specific IgM antibodies against B. burgdorferi. Consequently, the test results of samples 32 and 62 point to an infection with B. burgdorferi. The distribution of immunoblot bands reported for the positive sample 62 is depicted below in Figure 4 [Fig. 4]. Figure 5 [Fig. 5] shows the distribution of the reported immunoblot bands as obtained by the different manufacturers’ assays [6], [7]. The overall diagnostic evaluation of the negative samples 31 and 61 was 98.2–99.7%, while it was 90.2–97.7% for the positive samples 32 and 62.
Figure 4: The distribution of the immunoblot bands reported for the positive sample 62 [7], [8]; recovery rate (%) of the submitted IgG immunoblot bands for sample 332/32 (May 2016), participants N=301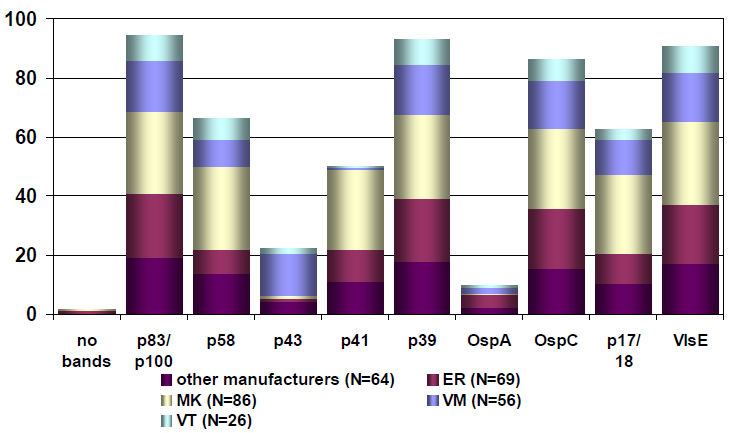
Figure 5: Distribution of the reported immunoblot bands as obtained by the different manufacturers’ assays [7], [8]; recovery rate (%) of the reported IgG immunoblot bands for sample 332/62 (Nov. 2016); participants N=311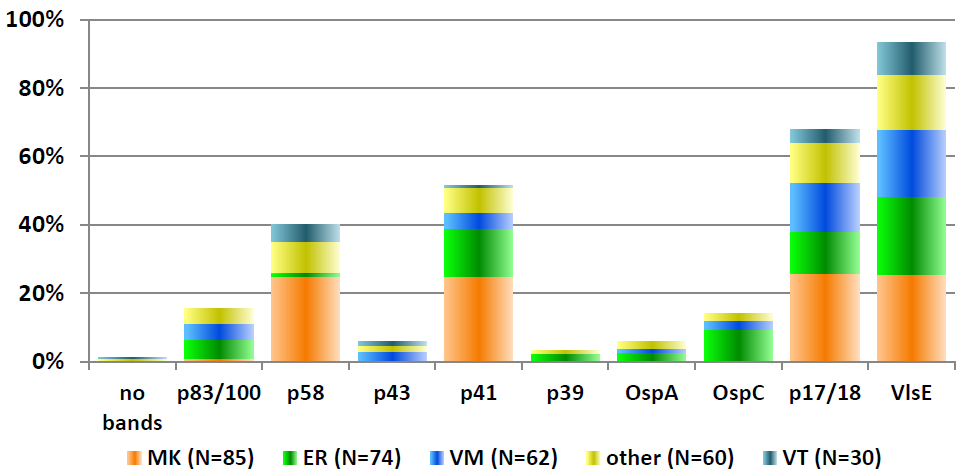
3.18 Helicobacter pylori antibodies (334)
3.18.1 Sample information
Samples 31 and 62 originated from clinically healthy blood donors without evidence of an infection.
Samples 32 and 61 were taken from a helicobacter-positive patient after finishing eradication therapy.
3.18.2 Determination of the target values
The mode of the results of the reference laboratories was set as the target value for the qualitative test results. The corresponding target values, target ranges, test results and pass rates are shown in Table 18 [Tab. 18].
Table 18: Helicobacter pylori antibody detection during the proficiency testing trials 2016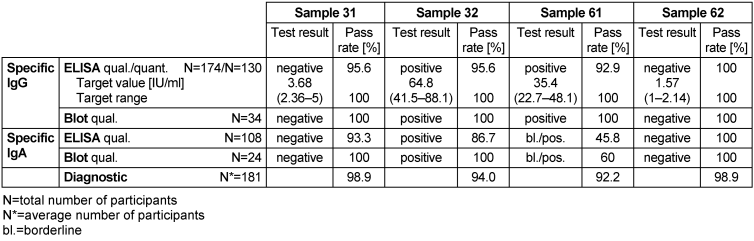
3.18.3 Overall diagnostic interpretation and commentary on the test results
No evidence of an infection with H. pylori was detected in samples 31 and 62. In sample 61, borderline results for IgA and positive results for IgG antibodies against the tested pathogen were detected, and in sample 32, positive results for IgA and IgG antibodies were detected, indicating a Helicobacter infection in both samples 32 and 61. Both expert laboratories and participants interpreted this constellation of results as pointing to a possible infection or colonization with H. pylori. In sample 61, the qualitative results for IgA antibodies for ELISA were 45.8% and for immunoblot they were 60%. The overall diagnostic evaluation of the negative samples 31 and 62 was 98.9%, while for the positive samples 32 and 61 it was 92.2–94.0%.
4 Discussion and conclusion
EQA is becoming increasingly important as a basis for developing strategies to improve diagnostic processes. The results of the EQA presented in this report generally show moderate to very good diagnostic quality. The average overall pass rate of the above-mentioned serological testing samples was over 90% in both proficiency testing trials. However, some special comments on serology need to be made:
In the case of the Treponema pallidum serology (311), the overall pass rate in the positive sample 61 for all test methods was 79.9%, indicating an unsatisfactory result. The lowest pass rate of 52.9% was seen in the quantitative detection of IgG antibodies by FTA-ABS. In the positive sample 31, the lowest pass rate of 58.3% was observed for the quantitative detection of IgG antibodies by FTA-ABS. In terms of the qualitative results of the negative sample 32, the lowest pass rate (57.1%) was observed for the ELISA test. These results are striking and could be problematic in the context of blood donor screening or screening during pregnancy. Regarding Chlamydia trachomatis serology (312), the overall pass rate was between 33.3% and 100% for all tests in sample 61. A pass rate of 33.3% was observed for the qualitative detection of IgA antibodies by immunoblot. With respect to the Coxiella burnetii serology (325), in the diagnostic assessment of the positive sample 61, the overall pass rate was between 73.8% and 100% for all test methods with the lowest pass rates for IgG or IgM analysis by IFT. In the case of the Borrelia burgdorferi serology (332), an overall pass rate of 97.7% was recorded for all test methods in the diagnostic assessment of the positive sample 32. The lowest pass rate of 63.6% was observed in the quantitative detection of IgG antibodies for IFT. In the Helicobacter pylori serology (334), the overall pass rate for sample 61 was between 45.8% and 100%. However, in terms of the qualitative result, the pass rate was 45.5% for ELISA and 60% for immunoblot, but only with respect to IgA antibody detection.
The above-mentioned findings and comments show the need for further improvement in certain diagnostic procedures in order to achieve more standardized and higher quality testing in Germany. This would ensure even better diagnostic test results in the routine clinical setting.
Notes
Competing interests
The authors declare that they have no competing interests.
References
[1] Wellinghausen N, Abele-Horn M, Donoso Mantke O, Enders M, Fingerle V, Gärtner B, Hagedorn J, Rabenau HF, Reiter-Owona I, Tintelnot K, Weig M, Zeichhardt H, Hunfeld KP. MiQ 35a–c Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik: Infektionsimmunologische Methoden Teil 1–3. München, Jena: Urban & Fischer; 2016.[2] Mai M, Müller I, Hunfeld KP. Qualität bakteriologisch-infektionsserologischer Verfahren in Deutschland: Auswertung der infektionsserologischen Ringversuche 2011 – Beitrag der Qualitätssicherungskommission der Deutschen Gesellschaft für Hygiene und Mikrobiologie (DGHM). GMS Z Forder Qualitatssich Med Lab. 2014;5:Doc04. DOI: 10.3205/lab000014
[3] Bundesärztekammer (German Medical Association); Instand e.V. Guidelines of the German Medical Association on quality assurance in medical laboratory testing. GMS Z Forder Qualitatssich Med Lab. 2015;6:Doc03. DOI: 10.3205/lab000018
[4] Müller I, Besier S, Hintereder G, Brade V, Hunfeld KP. Zur Qualität der bakteriologischen Infektionsserologie in Deutschland: eine Metaanalyse der infektionsserologischen Ringversuche des Jahres 2006 – Beitrag der Qualitätssicherungskommission der DGHM. GMS Z Forder Qualitatssich Med Lab. 2009;1:Doc04. DOI: 10.3205/lab000004
[5] Müller I, Hunfeld KP. Manual May 2016 Testing Information Bacteriology Infection Serology DAkkS. D-EP-15027-02-00 – May 2016. Düsseldorf: INSTAND e.V.; 2016 [accessed 2020 Jan 20]. Available from: https://www.instand-ev.de/uploads/tx_nfextinstandpdf/Begleitheft_Mai_eng_01.pdf
[6] Müller I, Hunfeld KP. Report on INSTAND e.V. EQAS 310-334 – May 2016. Düsseldorf: INSTAND e.V.; 2016 [accessed 2020 Jan 20]. Available from: https://www.instand-ev.de/uploads/tx_nfextinstandpdf/RV_310-334_Mai_en_01.pdf
[7] Müller I, Hunfeld KP. Report on INSTAND e.V. EQAS 310-334 – November 2016. Düsseldorf: INSTAND eV; 2016 [accessed 2020 Jan 20]. Available from: https://www.instand-ev.de/System/rv-files/RV_310-334_Nov_en.pdf




