[Kombinierte kognitive Behandlung bei Patienten mit krebsbedingter kognitiver Dysfunktion – vorläufige Daten aus einer Pilotstudie]
Oliver Rick 1Maren Schäfer 1
Alena Garber 1
1 Klinik Reinhardshöhe, Bad Wildungen, Germany
Zusammenfassung
Eine krebsbedingte kognitive Dysfunktion (CRCD) tritt bei bis zu 60% der Patienten nach einer Tumortherapie auf und beeinträchtigt die Lebensqualität oft erheblich.
Die Daten von onkologischen Patienten mit CRCD, die im Rahmen der stationären onkologischen Rehabilitation an einem kombinierten kognitiven Training teilnahmen, wurden retrospektiv analysiert. Die Patienten erhielten ein webbasiertes kognitives Training in Kombination mit einer verhaltenstherapeutischen Intervention. Achtunddreißig Patienten wurden in die Studie aufgenommen. In der subjektiven Wahrnehmung der Patienten zeigte sich eine signifikante Verbesserung der kognitiven Funktion durch die kombinierte kognitive Therapie. Auch beim kognitiven Screening ergaben sich signifikante Verbesserungen, insbesondere bei den Parametern Wachsamkeit und selektive Aufmerksamkeit/Reaktionskontrolle. Darüber hinaus wurde ein signifikanter Zusammenhang zwischen der subjektiven Wahrnehmung und der Aufmerksamkeit sowie dem Arbeitsgedächtnis am Ende der Therapie festgestellt. Eine kombinierte kognitive Therapie könnte sowohl die subjektiv wahrgenommene als auch die objektiv gemessene kognitive Funktion im Rahmen der stationären onkologischen Rehabilitation verbessern. Eine randomisierte Studie ist notwendig und geplant.
Introduction
Cancer-related cognitive dysfunction (CRCD) is a short-term, long-term or permanent functional impairment of attention, memory, processing speed and the ability to perform complex tasks [1]. Depending on the tumor disease under investigation, the prevalence of CRCD varies between 12–68% [2]. According to subjective patient reports, this figure can increase to 80% 6 months after the end of chemotherapy [3]. CRCD after oncological therapy is described by patients up to 20 years after cancer treatment [4]. It can therefore hinder activities of daily living and the resumption of professional activities in the long term or permanently [5]. Pathophysiologically, CRCD is most likely a multifactorial process. In addition to the actual tumor disease, genetic conditions and antitumor therapy play a significant role. The interaction of these three components ultimately determines the severity of the cognitive dysfunction [6]. Drug-based tumor therapy in particular is associated with inflammatory processes and secondary changes such as vascular damage, hormonal and metabolic changes [7]. However, a large multi-center study with 477 patients with early or locally advanced breast cancer showed that the administration of chemotherapy has no significant influence on the development of CRCD. On the contrary, symptoms of latent depression are associated with significantly higher rates of CRCD [8]. Systematic screening of cognitive disorders has not yet been established, but can be routinely assessed primarily with the numerical analogue scale (NRS) and subsequently confirmed with a validated questionnaire such as the FACT-Cog (Functional Assessment of Cancer Therapy – Cognitive Function), the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QoL30) or the Fragebogen erlebter Defizite der Aufmerksamkeit (Questionnaire of Subjectively Experienced Deficits of Attention, FEDA) [9]. Routine neuropsychological test batteries are rarely used in everyday clinical practice outside of studies, as they are associated with high personnel and time expenditure [10]. The International Cognition and Cancer Task Force (ICCTF) recommends assessing learning, memory, processing speed and executive functions [5]. Validated neuropsychological tests such as the Hopkins Verbal Learning Test-Revised (HVLT-R), the Trail Making Test (TMT) and the Attention Load Test (d2 test) are available for this purpose [11]. The classification into three severity levels can be made according to the Common Terminology Criteria for Adverse Events (CTCAE Version 4.0) of the National Cancer Institute (NCI). In the last 10 years, research into measures to improve CRCD has led to a large number of systematic reviews and meta-analyses [12]. Web-based cognitive training, cognitive rehabilitation, mindfulness techniques and physical activity appear to be the most effective [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. In a systematic review of 19 studies on cognitive rehabilitation, 13 intervention programs, either computer-based training or compensatory strategy training, were evaluated in 1,124 cancer survivors. All studies reported improvements in at least one self-reported or objective cognitive measure. In 87% (13/15) of controlled trials, improvement was seen in objective measures, particularly memory, executive function and processing speed. In ten of 16 studies (63%) with longer follow-up, the effects of the intervention were found to persist for at least 2 months after the intervention. Effect sizes varied from small to large [23]. Although cognitive rehabilitation programs are routinely used in other clinical populations with acquired cognitive impairment, they are not routinely offered to cancer survivors with CRCD. Furthermore, it is currently only rudimentarily known which type of treatment intervention provides the greatest benefit. A recent three-arm randomized controlled trial recruited 65 women with breast cancer following adjuvant chemotherapy. They received either attention process training (APT), compensatory strategy training (CST) or observation only. Active interventions included 6-week, 2-hour/week small group sessions. Cognitive performance was measured using the FACT-COG-PCI subscale, neuropsychological testing and a functional impact analysis (FIA) before and after the intervention and 6 and 12 months later. Although there was a slight decline in cognitive dysfunction, the difference between the intervention groups and the control group was not significant [24].
Due to the paucity of data with predominantly negative results on psychological interventions, we conducted a retrospective analysis of behavior-oriented cognition therapy in everyday life (KIA program) in the context of inpatient oncological rehabilitation. The cognition therapy was combined with web-based training. The aim of the study was to determine whether this combined treatment is tolerated by patients, is feasible in inpatient oncological rehabilitation and can be expected to be effective.
Materials and methods
Study design and data collection
This is a retrospective longitudinal study that was conducted during inpatient oncological rehabilitation at Klinik Reinhardshöhe, Bad Wildungen, Germany. In the period from July 2020 to April 2023, 38 cancer patients with CRCD (visual analog scale ≥5) were chronologically included. All patients were retrospectively informed by telephone about the data analysis and written informed consent was obtained. Patients were excluded if they did not consent to data analysis, did not receive web-based training and had difficulties in using the computer, suffered from a tumor of the central nervous system, had multiple comorbidities or a psychiatric illness, had received whole-brain radiotherapy, or were not sufficiently proficient in the German language.
As this was a retrospective analysis, no ethics vote is available. The current version of the Declaration of Helsinki was adhered to.
Diagnostic assessment
On admission to clinic, patients were asked about the presence of CRCD using the visual analog scale (VAS) with a scale of 0–10. From a value of ≥5, a computer-based screening was carried out using RehaCom® [16]. This was carried out in individual sessions in a separate room so that the patients were protected from external disruptive factors, such as increased background noise. In addition, the patients were given the FEDA for self-assessment of attention deficits [25]. The diagnostics were repeated at the end of rehabilitation in order to measure the therapeutic effect.
Therapeutic intervention
In addition to web-based training (RehaCom®, HASOMED GmbH, Paul-Ecke-Straße 1, 39114 Magdeburg), behavior-oriented cognitive therapy (KIA program) was used to treat CRCD. This includes three therapy sessions in interactive small groups to impart knowledge about CRCD, teach different learning types and teach memory techniques in everyday life. In a further group session, the patients’ individual workplace situations were discussed. The extent to which stress at work could be reduced and mental recovery phases could be used when cognitive performance declines was discussed together. In addition, the patients were recommended a daily exercise program and participation in mindfulness training at least once a week. The latter included either Qigong, yoga, mindfulness-based stress reduction (MBSR), progressive muscle relaxation according to Jacobson or a combination of these.
Data collection and data analysis
Data was collected by reviewing the medical records of patients who complained of CRCD during their inpatient oncological rehabilitation and came to occupational therapy for cognitive training. The patients were informed about the study, taking into account the inclusion and exclusion criteria mentioned above. After giving verbal consent to the study, the patients received a written consent form and were included in the study after signing it. The data were analyzed using SPSS Statistics 26 (SPSS Inc., an IBM Company, Chicago, IL). Non-parametric tests were used to examine the questions, as the sample was not large enough to assume a normal distribution. The Wilcoxon test to measure possible differences between two dependent samples and the Mann-Whitney U test to measure possible differences between two independent samples were used to answer the various questions. In addition, the Kruskal-Wallis test was used to measure possible differences between several independent samples and Spearman’s rank correlation coefficient was used to assess a potential correlation between two at least ordinal scaled variables. A significance level of p≤0.05 was assumed.
Results
A total of 38 patients was included in the study. The mean age was 53 years (range: 30–60 years) and the mean length of stay in the clinic was 25 days (range: 18–35 days). A good 2/3 of the patients were women and came to the clinic as part of a follow-up rehabilitation program. Almost 90% of the patients still had an existing job, and 33/38 patients (87%) were unable to work at the time of admission. The patients were suffering from either breast cancer or bronchial or colon carcinoma in the predominantly local or locally advanced tumor stage. Chemotherapy had been given to 23/38 patients (60%). With regard to therapy, all patients received web-based cognitive training, tasks that were completed using paper pencils and 29/38 patients (76%) also received the KIA program. While a sports program was used by almost all patients (97%), mindfulness training was only used by a smaller number of patients (Table 1 [Tab. 1]).
Table 1: Patient characteristics (n=38)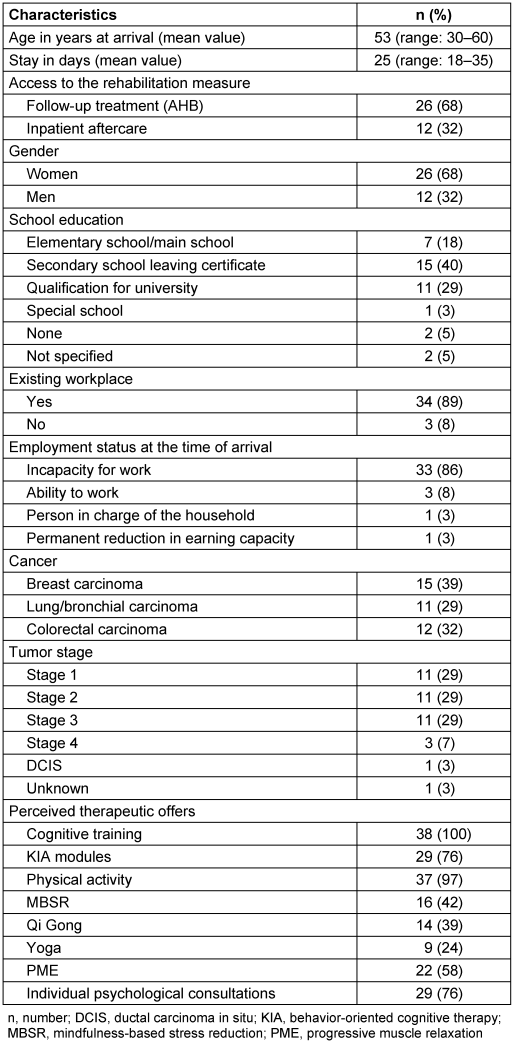
Subjective perception
The subjective perception of CRCD experienced before and after therapeutic intervention was compared. The measurements showed that with regard to the subjective assessment using the FEDA questionnaire, the average score had increased overall for all patients at the end of therapy (72.9 points versus 89.7 points). On average, the FEDA score was significantly higher with an increase of 17 points. The subgroups of the FEDA questionnaire “Distractibility and slowing down of mental processes”, “Fatigue and slowing down during practical activities” and “Reduced drive” showed significantly higher values on average at the end of therapy (p=0.00) (Table 2 [Tab. 2]).
Table 2: Subjective assessment of cognitive function using the FEDA questionnaire (n=38)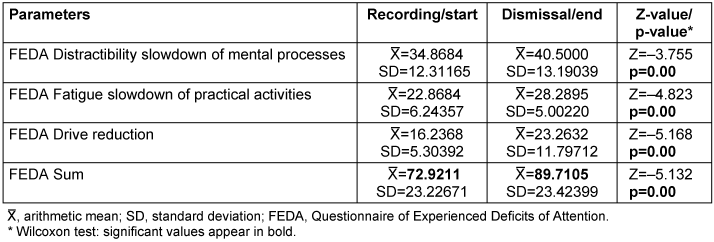
Cognitive screening
The objective test results using cognitive screening in 29 patients also showed a significant improvement between the beginning and end of therapy. Statistically relevant changes were found in the parameters “Alertness without warning tone” (p=0.025), “Alertness with warning tone” (p=0.018) and the parameter “Selective attention/reaction control” (p=0.03). A positive trend was found in the working memory parameter (p=0.063) (Table 3 [Tab. 3]).
Table 3: Objective measurement of cognitive function in cognitive screening (n=29)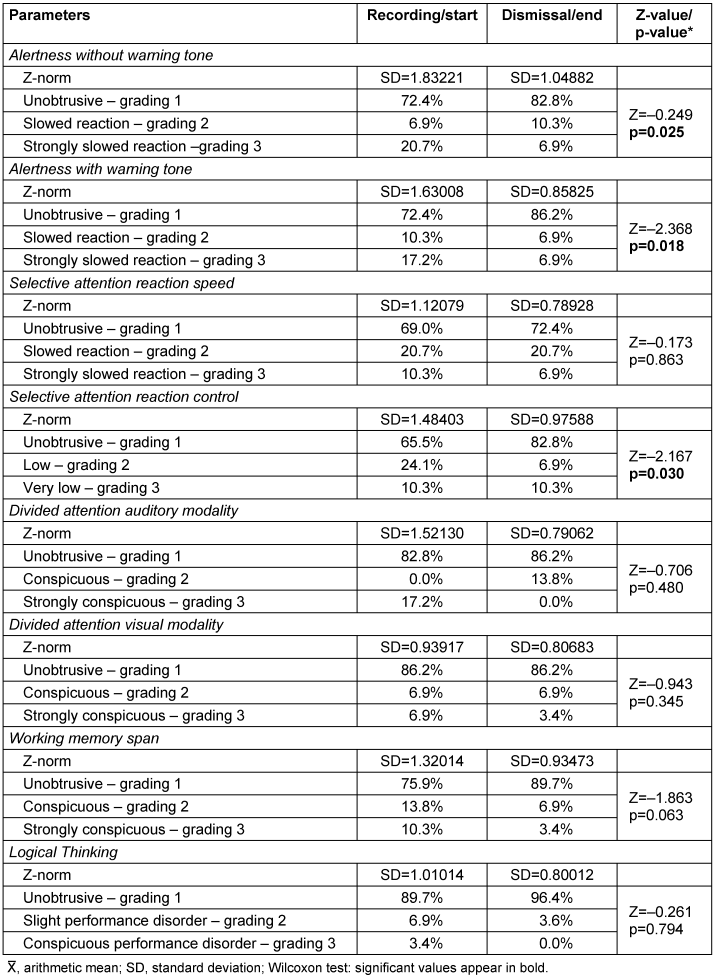
Correlation between subjective perception and cognitive screening
The subjective information from the FEDA questionnaire was correlated with the measured values from the cognitive screening (Table 4 [Tab. 4]). The results show no statistically significant correlations between the variables subjective assessment and objective measurement at the start of rehabilitation. Weak positive correlations were found between the parameters “Distractibility and slowing down of mental processes” and “Alertness without warning tone” (p=0.06). The parameters “Distractibility and slowing of mental processes” and “Divided attention/auditory modality” as well as “Divided attention/visual modality” were equally weakly correlated with each other (p=0.06 and p=0.10). The FEDA sum at the beginning of therapy also correlated weakly with the parameter “Divided attention/auditory modality” (p=0.07).
Table 4: Correlations between the subjective assessment and the objective measurement at the start of therapy (n=29)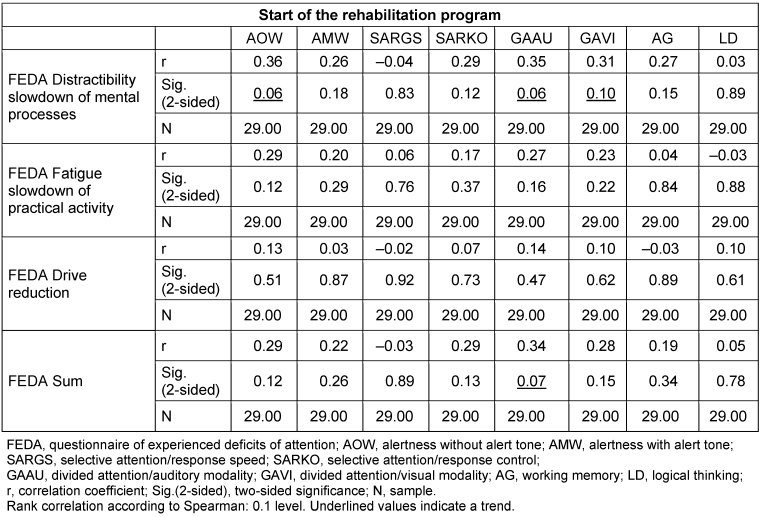
The measured values at the end of therapy are shown in Table 5 [Tab. 5]. Statistically significant correlations were found between the subjective parameter of the FEDA questionnaire “Distractibility and slowing down of mental processes” and the objective parameters “Alertness without warning tone” (p=0.00) and “Alertness with warning tone” (p=0.03) of the cognitive screening. Similar results were found between the FEDA final sum and the aforementioned screening parameters (p=0.01 and p=0.04). The subgroup “fatigue and slowing down of practical activities” correlated equally with the parameter “alertness without warning tone” (p=0.01). The parameter “working memory/memory span” correlated with all parameters of the FEDA questionnaire (p=0.00, p=0.03 and p=0.01), with the exception of the parameter “reduction in drive”. There were no correlations between the parameters selective attention, divided attention and logical reasoning of the cognitive screening and the FEDA subjective self-assessment questionnaire.
Table 5: Correlations between the subjective assessment and the objective measurement at the end of therapy (n=29)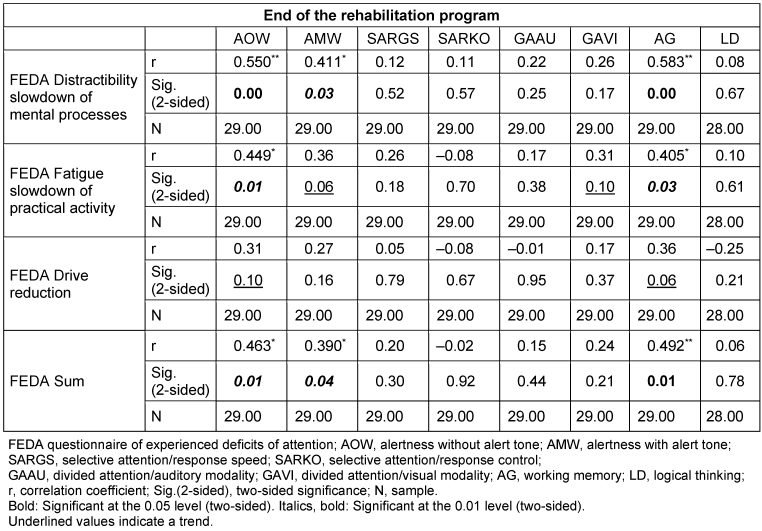
Discussion
The preliminary data from our pilot study suggest that the combination of behavioral cognitive therapy in daily life (KIA program) and web-based cognitive training could lead to an improvement in general attention and in the ability to concentrate in terms of selective attention/reaction control in cognitive screening. In addition, a subjective improvement in cognitive function was demonstrated using the FEDA questionnaire.
Our results are supported by previously published data. Von Ah et al. conducted a three-arm, single-blind randomized trial of cognitive training in 82 breast cancer patients who had received an average of 6 years of cancer therapy. The study treatment included either group sessions for memory training, web-based cognitive training, or no training via a waiting list. The authors found that training memory and processing speed improved objective cognitive performance. Subjective cognitive function, mood, anxiety, fatigue, and quality of life were also significantly improved in the cognitive training groups [26]. Furthermore, the results of the present study are consistent with those of Kesler et al. They conducted a randomized controlled trial to examine the feasibility and efficacy of a web-based training program in 62 female patients six years after breast cancer treatment. A total of 41 patients completed the training program over a period of 12 weeks, while 21 patients were on the waiting list. Compared to the patients on the waiting list, cognitive training significantly improved cognitive flexibility and processing speed, with marginally significant improvements in verbal memory. Self-assessment of cognitive abilities, including planning, organization, and task monitoring, was also better in the active group than in the waiting group [13]. In the study group we examined, the cognitive screening parameters showed an improvement in attention performance. The reaction speed and the general information processing speed (IPS) under increased vigilance and concentration (selective attention) showed overall improvements as a result of the cognitive training. This is consistent with the data from Kesler et al. [13], who also found an improved IPS as part of attention performance.
At the beginning of the therapy conducted by us, no statistically significant correlations were found between the objective measurement in the cognitive screening with RehaCom® and the subjectively experienced CRCD. At the end of therapy, correlations were found between some parameters of cognitive screening and subjective assessment using the FEDA questionnaire. The results suggest that patients’ assessment of their cognition at the end of therapy was more realistic than at the beginning, as there may have been a bias between subjective assessment and objective measurement of cognition at the beginning of therapy. However, there is no evidence for this in the literature so far.
In a recently published study by Vardy et al. [24], which also examined behavioral cognitive therapy, no benefits in terms of cognitive function were found. 65 cancer survivors, predominantly with breast cancer, with cognitive symptoms 6–60 months after adjuvant chemotherapy were randomized to either an attention training (APT) and compensatory strategy (CST) or a control group. The active interventions consisted of 6-week small-group sessions lasting 2 hours per week. Cognitive function was assessed before and after the intervention, 6 and 12 months later. The primary outcome measure was the change in cognitive symptoms on the FACT-COG-PCI subscale between baseline and after the intervention. Secondary outcomes included objective neuropsychological performance, functional impact analysis (FIA), and patient-reported outcomes and associations. Although subjective perception in the FACT-COG-PCI, clinical neuropsychological T-scores, and FIA improved over time in all groups, no significant differences were found between the two groups [24].
This is in contrast to the most recent data from Klaver et al. [27]. A cognitive rehabilitation program that includes self-management, psychoeducation, fatigue management, coping with the consequences of cognitive problems, and communication strategies (basic program) was compared to an extended program (basic program plus strategy training). There was also a control group with a waiting list. In the three-armed randomized controlled trial, 279 patients with tumors outside the CNS and cognitive complaints were assigned to either the basic program (n=93), the extended program (n=93), or the control group (n=93). Participants completed measurements before randomization (T0), 12 weeks after randomization at the end of the program (T1), and 26 weeks after randomization (T2). In all three groups, statistically significant improvements in goal achievement were observed over time. Participants in the extended program were found to achieve their pre-set goals significantly better than the control group participants, both at short-term (T0–T1) and long-term (T0–T2) follow-up (p=0.014). Participants in the basic program did not show significantly better goal achievement than the control group at either short-term or long-term follow-up (p=0.92) [27].
In summary, based on our preliminary data and the data from the literature to date, it can be assumed that the combination of web-based cognitive training and a behavioral cognitive therapy program could be an effective treatment for CRCD. However, a randomized controlled trial is needed to confirm the effectiveness of this form of therapy. The data collected here as part of the pilot study will be used to plan such a study.
Notes
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Competing interests
The authors declare that they have no competing interests.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013.
References
[1] Ganz PA, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman DH, Cole SW, Irwin MR, Ancoli-Israel S, Belin TR. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013 Jun;105(11):791-801. DOI: 10.1093/jnci/djt073[2] Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog – methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Res Treat. 2006 Jan;95(2):125-9. DOI: 10.1007/s10549-005-9055-1
[3] Kohli S, Griggs JJ, Roscoe JA, Jean-Pierre P, Bole C, Mustian KM, Hill R, Smith K, Gross H, Morrow GR. Self-reported cognitive impairment in patients with cancer. J Oncol Pract. 2007 Mar;3(2):54-9. DOI: 10.1200/JOP.0722001
[4] Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012 Apr;30(10):1080-6. DOI: 10.1200/JCO.2011.37.0189
[5] Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, Winocur G, De Ruiter MB, Castel H. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019 Dec;30(12):1925-40. DOI: 10.1093/annonc/mdz410
[6] Lange M, Joly F. How to Identify and Manage Cognitive Dysfunction After Breast Cancer Treatment. J Oncol Pract. 2017 Dec;13(12):784-90. DOI: 10.1200/JOP.2017.026286
[7] Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008 Apr;19(4):623-9. DOI: 10.1093/annonc/mdm500
[8] Rick O, Reuß-Borst M, Dauelsberg T, Hass HG, König V, Caspari R, Götz-Keil G, Pfitzner J, Kerschgens C, Fliessbach K, Hoppe C. NeuroCog FX study: A multicenter cohort study on cognitive dysfunction in patients with early breast cancer. Psychooncology. 2018 Aug;27(8):2016-22. DOI: 10.1002/pon.4763
[9] Rick O, Gerhardt A, Schilling G. Cancer-Related Cognitive Dysfunction: A Narrative Review for Clinical Practice. Oncol Res Treat. 2024;47(5):218-23. DOI: 10.1159/000538277
[10] Duivon M, Lange M, Binarelli G, Lefel J, Hardy-Léger I, Kiasuwa-Mbengi R, Méric JB, Charles C, Joly F. Improve the management of cancer-related cognitive impairment in clinical settings: a European Delphi study. J Cancer Surviv. 2024 Dec;18(6):1974-97. DOI: 10.1007/s11764-023-01436-8
[11] European Cancer and Cognition Consortium (ECCC), Sleurs C, Amidi A, Wu LM, Kiesl D, Zimmer P, Lange M, Rogiers A, Giffard B, Binarelli G, Borghgraef C, Deprez S, Duivon M, De Ruiter M, Schagen S, Ahmed-Lecheheb D, Castel H, Buskbjerg CR, Dos Santos M, Joly F, Perrier J. Cancer-related cognitive impairment in non-CNS cancer patients: Targeted review and future action plans in Europe. Crit Rev Oncol Hematol. 2022 Dec;180:103859. DOI: 10.1016/j.critrevonc.2022.103859
[12] Binarelli G, Joly F, Tron L, Lefevre Arbogast S, Lange M. Management of Cancer-Related Cognitive Impairment: A Systematic Review of Computerized Cognitive Stimulation and Computerized Physical Activity. Cancers (Basel). 2021 Oct;13(20):5161. DOI: 10.3390/cancers13205161
[13] Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, Mustian K, Morrow G. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013 Aug;13(4):299-306. DOI: 10.1016/j.clbc.2013.02.004
[14] Ercoli LM, Petersen L, Hunter AM, Castellon SA, Kwan L, Kahn-Mills BA, Embree LM, Cernin PA, Leuchter AF, Ganz PA. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology. 2015 Nov;24(11):1360-7. DOI: 10.1002/pon.3769
[15] Bray VJ, Dhillon HM, Bell ML, Kabourakis M, Fiero MH, Yip D, Boyle F, Price MA, Vardy JL. Evaluation of a Web-Based Cognitive Rehabilitation Program in Cancer Survivors Reporting Cognitive Symptoms After Chemotherapy. J Clin Oncol. 2017 Jan;35(2):217-25. DOI: 10.1200/JCO.2016.67.8201
[16] Dos Santos M, Hardy-Léger I, Rigal O, Licaj I, Dauchy S, Levy C, Noal S, Segura C, Delcambre C, Allouache D, Parzy A, Barriere J, Petit T, Lange M, Capel A, Clarisse B, Grellard JM, Lefel J, Joly F. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: A 3-arm randomized trial. Cancer. 2020 Dec;126(24):5328-36. DOI: 10.1002/cncr.33186
[17] Vadiraja SH, Rao MR, Nagendra RH, Nagarathna R, Rekha M, Vanitha N, Gopinath SK, Srinath B, Vishweshwara M, Madhavi Y, S Ajaikumar B, Ramesh SB, Rao N. Effects of yoga on symptom management in breast cancer patients: A randomized controlled trial. Int J Yoga. 2009 Jul;2(2):73-9. DOI: 10.4103/0973-6131.60048
[18] Milbury K, Chaoul A, Biegler K, Wangyal T, Spelman A, Meyers CA, Arun B, Palmer JL, Taylor J, Cohen L. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psychooncology. 2013 Oct;22(10):2354-63. DOI: 10.1002/pon.3296
[19] Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, Lee MS, Rosenthal DS, Larkey L, Vardy J. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2012 Jun;20(6):1235-42. DOI: 10.1007/s00520-011-1209-6
[20] Campbell KL, Zadravec K, Bland KA, Chesley E, Wolf F, Janelsins MC. The Effect of Exercise on Cancer-Related Cognitive Impairment and Applications for Physical Therapy: Systematic Review of Randomized Controlled Trials. Phys Ther. 2020 Mar;100(3):523-542. DOI: 10.1093/ptj/pzz090
[21] Johns SA, Von Ah D, Brown LF, Beck-Coon K, Talib TL, Alyea JM, Monahan PO, Tong Y, Wilhelm L, Giesler RB. Randomized controlled pilot trial of mindfulness-based stress reduction for breast and colorectal cancer survivors: effects on cancer-related cognitive impairment. J Cancer Surviv. 2016 Jun;10(3):437-48. DOI: 10.1007/s11764-015-0494-3
[22] Janelsins MC, Peppone LJ, Heckler CE, Kesler SR, Sprod LK, Atkins J, Melnik M, Kamen C, Giguere J, Messino MJ, Mohile SG, Mustian KM. YOCAS©® Yoga Reduces Self-reported Memory Difficulty in Cancer Survivors in a Nationwide Randomized Clinical Trial: Investigating Relationships Between Memory and Sleep. Integr Cancer Ther. 2016 Sep;15(3):263-71. DOI: 10.1177/1534735415617021
[23] Fernandes HA, Richard NM, Edelstein K. Cognitive rehabilitation for cancer-related cognitive dysfunction: a systematic review. Support Care Cancer. 2019 Sep;27(9):3253-79. DOI: 10.1007/s00520-019-04866-2
[24] Vardy JL, Pond GR, Bell ML, Renton C, Dixon A, Dhillon HM. A randomised controlled trial evaluating two cognitive rehabilitation approaches for cancer survivors with perceived cognitive impairment. J Cancer Surviv. 2023 Dec;17(6):1583-95. DOI: 10.1007/s11764-022-01261-5
[25] Sturm W. Aufmerksamkeitsstörungen. Hogrefe: Göttingen; 2005. (Fortschritte der Neuropsychologie; 4).
[26] Von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M, Rebok G, Ball K, Schneider B, Weaver M, Tallman E, Unverzagt F. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012 Oct;135(3):799-809. DOI: 10.1007/s10549-012-2210-6
[27] Klaver KM, Duijts SFA, Geusgens CAV, Kieffer JM, Agelink van Rentergem J, Hendriks MP, Nuver J, Marsman HA, Poppema BJ, Oostergo T, Doeksen A, Aarts MJB, Ponds RWHM, van der Beek AJ, Schagen SB. Internet-based cognitive rehabilitation for working cancer survivors: results of a multicenter randomized controlled trial. JNCI Cancer Spectr. 2024 Jan;8(1):pkad110. DOI: 10.1093/jncics/pkad110




