Prospective data collection and analysis of perforations and tears of latex surgical gloves during primary endoprosthetic surgeries
Sarah Zaatreh 1Andreas Enz 1
Annett Klinder 1
Tony König 1
Lena Mittelmeier 1
Günther Kundt 2
Wolfram Mittelmeier 1
1 Department of Orthopedics, University Medicine Rostock, Rostock, Germany
2 Institutes for Biostatistics and Informatics in Medicine and Ageing Research, University of Rostock, Rostock, Germany
Abstract
Introduction: Surgical gloves are used to prevent contamination of the patient and the hospital staff with pathogens. The aim of this study was to examine the actual effectiveness of gloves by examining the damage (perforations, tears) to latex gloves during surgery in the case of primary hip and knee prosthesis implantation.
Materials and methods: Latex surgical gloves used by surgeons for primary hip and knee replacement surgeries were collected directly after the surgery and tested using the watertightness test according to ISO EN 455-1:2000.
Results: 540 gloves were collected from 104 surgeries. In 32.7% of surgeries at least one glove was damaged. Of all the gloves collected, 10.9% were damaged, mainly on the index finger. The size of the perforations ranged from ≤1 mm to over 5 mm. The surgeon’s glove size was the only factor that significantly influenced the occurrence of glove damage. Surgeon training level, procedure duration, and the use of bone cement had no significant influence.
Conclusions: Our results highlight the high failure rate of surgical gloves. This has acute implications for glove production, surgical practice, and hygiene guidelines. Further studies are needed to detect the surgical steps, surface structures, and instruments that pose an increased risk for glove damage.
Keywords
surgical gloves, latex, perforations, tears, orthopedic surgery, ISO DIN 455-1, joint replacement, infection risk
Background
Intact surgical gloves function as an important barrier against the transmission of bacteria between the surgical team and their patient [1], [2], [3], [4]. Cross contaminations and surgical site infections are a major risk in orthopedic surgery [5], [6], [7], [8], [9], [10]. Although there are now strict guidelines regarding the prevention of such complications, the number of infections is still high. Besides perioperative application of antibiotics and sterile conditions, the use of surgical gloves is supposed to prevent contamination of the patient and the hospital staff [11], [12], [13]. Yet trials have confirmed that perforations and tears of gloves are common [4], [5], [8], [9].
However, there is no standardized changing system specified for gloves during operations. The ISO EN 455-1:2000 [14] merely requires impermeableness and tear resistance as a prerequisite for gloves. However, the highly repetitive pressure and shear strain of the surgical routine are not reflected. Likewise, punctures resulting from sharp bone surfaces of implants, instruments, or cement surfaces are not taken into consideration [5].
The aim of this study was to evaluate the damage to latex gloves, which occurs during the implantation of prostheses in surgical orthopedics. The analysis followed the watertightness test according to ISO EN 455-1:2000. We compared the quantity and quality of glove damage in primary total knee and hip replacements.
Materials and methods
Study design and data collection
From May 1st, 2015 to May 1st, 2016 surgical gloves used during primary hip (ICD-9-CM code 81.51) and knee (ICD-9-CM code 81.54) replacement surgeries were collected at the Department of Orthopedics of the University Medicine Rostock. Throughout the study, 540 surgical gloves were retrieved from 104 elective primary endoprosthetic surgeries. Damage to surgical gloves was analyzed within 24 h after the surgery. The duration of the surgery, type of surgery (knee or hip), type of surgeon, number of used gloves, and glove size were recorded.
In the month after the collection period it was considered necessary to relate glove damage to some of the patient data. Therefore, we applied for ethical approval to access the patient’s data from their medical files that were unrelated to the study as part of standard clinical procedure. After ethical approval was given by the Local Ethical Committee of Rostock, Germany (registration number: A2016-0112), patient-related data (BMI, age, previous operation, date and type of operation) were collected retrospectively. From the 15 orthopedic surgeons participating in the study 14 were right-handed and one was left-handed. Seven were main surgeons and eight were surgeons in training. All surgeons expressed their consent to the examination of their gloves.
Surgical gloves
The standard gloves used during endoprosthetic surgery were sterile, powder-free disposable latex gloves for single use (ProtexisTM, Cardinal Health, Dublin, Ohio, USA). The size of the gloves is the hand circumference across the palm in inches. The surgical procedure at the Orthopedics Department of University Medicine Rostock involves the use of double gloves, one pair worn over the other. We refer to the pair worn over the first pair as the outer gloves.
Intraoperatively, exchanges of gloves were carried out when damage to the gloves was noticed during surgery. The changing process involved removing the glove and then turning it inside out.
Collection and integrity testing of the gloves
After an operation, all gloves (outer and inner) were safely kept. The samples were collected in a plastic bag, sealed, and labeled. The name of the patient, the patient’s date of birth, the name of the surgeon, and the type and date of the operation were recorded.
The investigation of micro perforations and tears of the surgical gloves was performed within 24 h by using the freedom from holes testing method described in the ISO EN 455-1:2000, Medical gloves for single use Part 1: Requirements and testing for freedom from holes, watertightness test [14]. A specifically manufactured watertightness measuring apparatus was made out of polycarbonate with two cylinders that have an outer diameter of 6 cm according to the technical drawing of ISO EN 455-1:2000. This apparatus was used to inspect the gloves for holes (Figure 1 [Fig. 1]). One glove was stretched over each of the cylinders up to a maximum of 4 cm and attached with a rubber seal to avoid slipping. Blood residues were removed carefully. By pulling each finger of the glove, the detection of even small damages was ensured. Thereafter, the gloves were filled with 1,000 ± 50 ml warm water (room temperature: 15–25°C). The stretched gloves were tested immediately and after an additional 3 min. Perforations or tears were confirmed when a drop of water or a jet leaked from the glove.
Figure 1: Watertightness tube apparatus to find perforations and tears via freedom from holes testing for used medical surgical gloves. A) Technical drawing of the watertightness tube / filling tube according to ISO EN 455-1:2000 [14]. B) Manufactured watertightness tube made out of polycarbonate with two cylinders of six centimeters outer diameter; glove was stretched over each of the cylinders up to a maximum of four centimeters and attached with a rubber seal.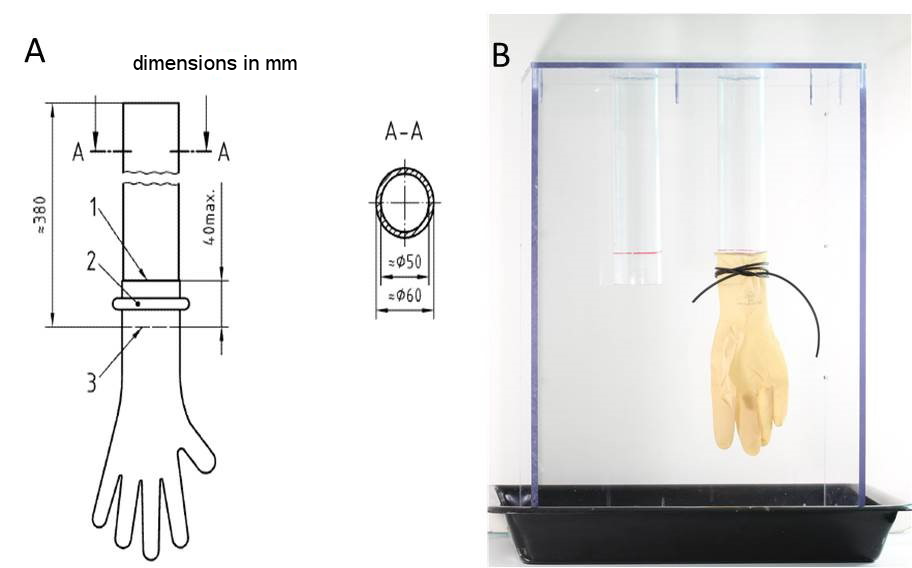
The dimensions of the perforations and tears were measured afterwards with a plastic goniometer (Kirchner & Wilhelm GmbH & Co. KG, Asperg, Germany) and the location of the damage was classified according to the finger on which it occurred. Overview images of the damages via 3D laser scanning microscopy (VK-X260, Keyence Corporation, Osaka, Japan) were also taken.
Statistical analysis
All data were stored and analyzed using the SPSS statistical package version 22 (IBM Corp., New York, USA). Descriptive statistics were computed for continuous and categorical variables. The statistics computed included means and standard deviations (SD) of continuous variables and are shown as mean ± SD or as frequencies [n] with percentages in brackets for categorical factors. For categorical factors, comparisons between patients with and without glove damage were performed by Fischer’s exact test (two categories) or by Pearson’s chi-squared test (more than two categories). Testing for differences of continuous variables between study groups created by existence of glove damage was accomplished by the two-sample t-test for independent samples or by the Mann-Whitney U-test by ranks as appropriate. Test selection was based on evaluating the variables for normal distribution, employing the Kolmogorov-Smirnov test. All p-values resulted from two-sided statistical tests and p≤0.05 was considered to be significant.
Results
General patient data and surgical procedure
A total of 540 surgical gloves were collected from the surgeries of 104 patients who participated in this study. Demographic data of the operated patients and the specific information about the surgical procedure are listed in Table 1 [Tab. 1]. The majority of gloves were collected from total hip replacement operations (74.1% total hip versus 25.9% total knee replacements) based on the given ratio in the study center. Bone cement was used in the majority of analyzed surgeries. In about one third of operations at least one damaged glove was identified, equaling 10.9% of all collected gloves.
Table 1: Overview of collected data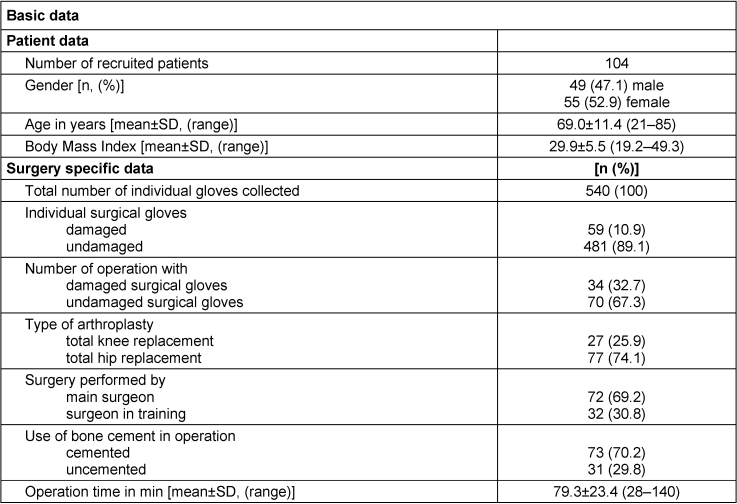
Association between patient data, operation specific data, and damage to surgical gloves
The factors analyzed for their influence on the occurrence of at least one damaged glove, are given in Table 2 [Tab. 2]. Mann-Whitney U-test by ranks, t-test for independent samples and Fischer’s exact test were employed to test for significance. The surgeon’s glove size was the only factor with a significant influence on the occurrence of at least one damaged glove (p=0.031). When glove damage occurred, the average glove size was approximately 7.8 compared to 8.0 where no damage occurred. The glove size is the circumference across the palm of the hand in inches.
Table 2: Statistical analysis of surgery data in relation to occurrence of glove damage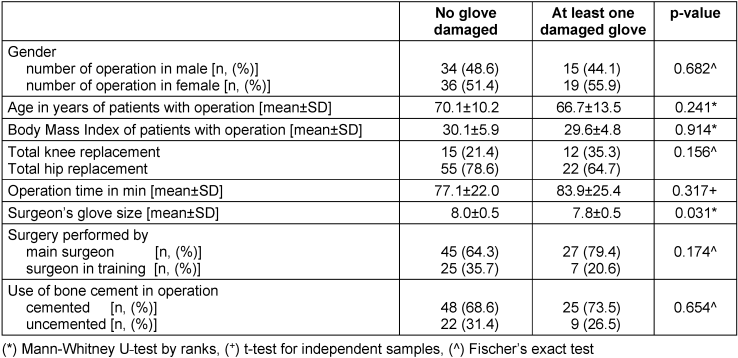
The data for the type of surgery (hip or knee), procedure duration, surgeon experience level (main or in training), use of bone cement showed no significant influence.
There was no association between the occurrence of damaged gloves and any of the patient data (gender, age, or BMI).
Position and dimension of the damage in surgical gloves
The highest incidence of glove damage occurred on the index finger at 62.7% (37/59) of all damaged gloves followed by the thumb, middle finger, and palm of the hand at 18.6% (11/59), 13.6% (8/59), and 5.1% (3/59) of all damaged gloves, respectively. No damage occurred on the ring finger or the little finger. Gloves from the dominant hand were more often affected by damage than gloves from the non-dominant hand (61.0% vs. 39.0%). When analyzing individual perforations, the incidence of damage was highest on the index finger of the dominant hand (39.0%) followed by the index finger of the non-dominant hand (23.7%), the thumb of the dominant hand (11.9%), and the middle finger of the dominant hand (8.5%). There was higher damage on the non-dominant hand (3.4%) compared to the dominant hand (1.7%) on the palm only. However, there was no significant difference in the distribution of the position of damage between the gloves on the dominant and non-dominant hands (p=0.795) (Table 3 [Tab. 3]).
Table 3: Comparison of position of glove damage between the dominant and the non-dominant hand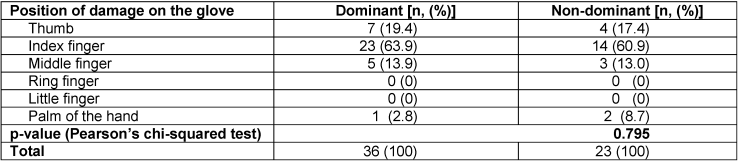
The dimensions of the damage ranged from ≤1 mm up to more than 5 mm. Most tears had a macroscopic size of 2 mm and 3 mm, however, microscopic tears smaller than 1 mm did also occur (Table 4 [Tab. 4]).
Table 4: Size of tears at the gloves in mm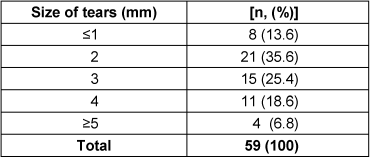
In order to characterize the type of damage to the gloves, 3D laser scanning microscopy was employed (Figure 2 [Fig. 2]). The types of damage we could identify were perforations, tears, and rubdown. In further studies, these images could be used to categorize the damage and correlate damage types to specific surgical steps.
Figure 2: 3D laser scanning microscopy images displaying different damage types of the surgical gloves A) 0.5 mm perforation at the right thumb. B) 3 mm tear at the right forefinger. C) 0.5 mm rubdown at the right middle finger. Images were taken at 20x magnification.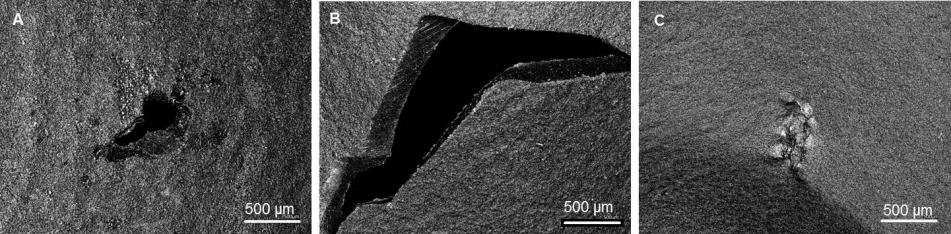
Discussion
With damage in surgical gloves occurring in more than 30% of the total joint replacement surgeries studied, there are a considerable proportion of surgeries in which the barrier function attributed to gloves is potentially compromised. The number of perforations and tears demonstrates the heavy strain on gloves in orthopedic surgery. In our study, we did not distinguish inner and outer gloves due to the fact that the benefits of double gloving have been well established and the required effort did not promise to add sufficient additional value [3], [4], [15], [16], [17].
Regarding the factor of surgery duration, the average duration of the procedures recorded in our study was already lower than the 90 minutes, which is recommended as the time after which gloves should be changed [18]. An average operation time of less than 90 minutes might also be the reason why in contrast to other studies, which report an increase in the perforation rate with the duration of glove wear [15], [18], we were unable to demonstrate a significant association between the occurrence of damage and the operation time.
The data on the location of the damage revealed that it was mainly the index finger that was affected. Contrary to the literature, in which the index finger of the non-dominant hand showed the highest perforation rate [15], [17], [18], we found that most of the damage occurred on the dominant hand. Further studies and analyses are needed to identify the specific steps, techniques, and instruments in surgery associated with an increased risk for glove damage. Chan et al. [19] found that damage to gloves in orthopedic procedures often occurred during the nailing procedure or internal fixings without wires and suggested that shear stress was responsible. Carter et al. [17], however, reported that most of the gloves were perforated in the period when the final components were implanted.
As demonstrated, 3D laser scanning microscopy can help to characterize and categorize different types of damage. This would allow further studies to link the type of damage to surgical procedures in order to identify causes of damage and work towards improving procedures and glove design. First developments in glove design were undertaken to prevent cross contamination even when the physical barrier is compromised by coating the inner lining of the glove with antimicrobial substances [20], [21]. However, the failure rates and observed damage highlight the importance of changing gloves as an immediate safety strategy and further studies are needed to estimate the efficiency of this strategy.
Conclusions
Surgical gloves are universally used to prevent contamination of patient and hospital staff with pathogens. Their reliability is of highest importance, but is not guaranteed. We believe that the incidence of damaged gloves in 30% of surgeries and the distinct locality of breaches necessitate an extension of the relevant ISO standards to include realistic strain tests. Specifically for the types of surgeries in this study, the assumed risk factors are: handling of hard and sharp bone, specific surgical instruments, surgical steps requiring increased use of force and risk of slipping. Our findings, in accordance with existing studies on surgical gloves, suggest a need for improvement of surgical procedures, glove handling guidelines, and glove design. Further studies are needed to detect the surgical steps and instruments that pose an increased risk for glove damage. Furthermore, guidelines for replacing gloves during surgery concerning exposure to patients’ bodily liquids and mechanically demanding steps of surgery should be modified with regard to national hygiene recommendations.
Notes
Trial identification number: A 2016-0112
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors developed the study and performed analysis of the data. SZ, AK and LM drafted the manuscript; all authors revised and approved the final version of the manuscript.
Acknowledgements
The authors want to thank the technical assistants Mr. Mario Jackzis and Ms. Laura Lux (Department of Orthopedics, University Medicine Rostock) for manufacturing the measuring apparatus and for imaging.
References
[1] Leitgeb J, Schuster R, Yee BN, Chee PF, Harnoss JC, Starzengruber P, Schäffer M, Assadian O. Antibacterial activity of a sterile antimicrobial polyisoprene surgical glove against transient flora following a 2-hours simulated use. BMC Surg. 2015 Jul;15:81. DOI: 10.1186/s12893-015-0058-5[2] Hübner NO, Goerdt AM, Stanislawski N, Assadian O, Heidecke CD, Kramer A, Partecke LI. Bacterial migration through punctured surgical gloves under real surgical conditions. BMC Infect Dis. 2010 Jul;10:192. DOI: 10.1186/1471-2334-10-192
[3] Guo YP, Wong PM, Li Y, Or PP. Is double-gloving really protective? A comparison between the glove perforation rate among perioperative nurses with single and double gloves during surgery. Am J Surg. 2012 Aug;204(2):210-5. DOI: 10.1016/j.amjsurg.2011.08.017
[4] Ersozlu S, Sahin O, Ozgur AF, Akkaya T, Tuncay C. Glove punctures in major and minor orthopaedic surgery with double gloving. Acta Orthop Belg. 2007 Dec;73(6):760-4.
[5] Timler D, Bończak O, Jończyk J, Iltchev P, Sliwczyński A, Marczak M. Risk assessment of accidental exposure of surgeons to blood during orthopedic surgery. Are we safe in surgical gloves? Ann Agric Environ Med. 2014;21(1):212-6.
[6] Yaldiz C, Yaldiz M, Ceylan N, Kacira OK, Ceylan D, Kacira T, Kizilcay G, Tanriverdi T. Retrospective, Demographic, and Clinical Investigation of the Causes of Postoperative Infection in Patients With Lumbar Spinal Stenosis Who Underwent Posterior Stabilization. Medicine (Baltimore). 2015 Jul;94(29):e1177. DOI: 10.1097/MD.0000000000001177
[7] Tanner J. Surgical gloves: perforation and protection. J Perioper Pract. 2006 Mar;16(3):148-52.
[8] Picheansanthian W, Chotibang J. Glove utilization in the prevention of cross transmission: a systematic review. JBI Database System Rev Implement Rep. 2015 May;13(4):188-230. DOI: 10.11124/jbisrir-2015-1817
[9] Pougnet R, Pougnet L, Garlantézec R. Comments Regarding Masroor et al: Perceptions and Barriers to Universal Gloving for Infection Prevention. Infect Control Hosp Epidemiol. 2016 Apr;37(4):488. DOI: 10.1017/ice.2016.6
[10] Harnoss JC, Brune L, Ansorg J, Heidecke CD, Assadian O, Kramer A. Practice of skin protection and skin care among German surgeons and influence on the efficacy of surgical hand disinfection and surgical glove perforation. BMC Infect Dis. 2014 Jun;14:315. DOI: 10.1186/1471-2334-14-315
[11] Harnoss JC, Kramer A, Heidecke CD, Assadian O. Wann sollte in Operationsräumen ein Wechsel chirurgischer Handschuhe erfolgen? [What is the appropriate time-interval for changing gloves during surgical procedures?]. Zentralbl Chir. 2010 Feb;135(1):25-7. DOI: 10.1055/s-0029-1224684
[12] Harnoss JC, Partecke LI, Heidecke CD, Hübner NO, Kramer A, Assadian O. Concentration of bacteria passing through puncture holes in surgical gloves. Am J Infect Control. 2010 Mar;38(2):154-8. DOI: 10.1016/j.ajic.2009.06.013
[13] Kramer A, Assadian O. Indications and the requirements for single-use medical gloves. GMS Hyg Infect Control. 2016;11:Doc01. DOI: 10.3205/dgkh000261
[14] DIN EN 455-1:2001-01: Medical gloves for single use - Part 1: Requirements and testing for freedom from holes; German version EN 455-1:2000. Beuth; 2001 [cited 2016 Oct 8]. Available from: https://www.beuth.de/de/norm/din-en-455-1/30945341
[15] Laine T, Aarnio P. How often does glove perforation occur in surgery? Comparison between single gloves and a double-gloving system. Am J Surg. 2001 Jun;181(6):564-6. DOI: 10.1016/S0002-9610(01)00626-2
[16] Yinusa W, Li YH, Chow W, Ho WY, Leong JC. Glove punctures in orthopaedic surgery. Int Orthop. 2004 Feb;28(1):36-9. DOI: 10.1007/s00264-003-0510-5
[17] Carter AH, Casper DS, Parvizi J, Austin MS. A prospective analysis of glove perforation in primary and revision total hip and total knee arthroplasty. J Arthroplasty. 2012 Aug;27(7):1271-5. DOI: 10.1016/j.arth.2012.01.021
[18] Partecke LI, Goerdt AM, Langner I, Jaeger B, Assadian O, Heidecke CD, Kramer A, Huebner NO. Incidence of microperforation for surgical gloves depends on duration of wear. Infect Control Hosp Epidemiol. 2009 May;30(5):409-14. DOI: 10.1086/597062
[19] Chan KY, Singh VA, Oun BH, To BH. The rate of glove perforations in orthopaedic procedures: single versus double gloving. A prospective study. Med J Malaysia. 2006 Dec;61 Suppl B:3-7.
[20] Assadian O, Kramer A, Ouriel K, Suchomel M, McLaws ML, Rottman M, Leaper D, Assadian A. Suppression of surgeons’ bacterial hand flora during surgical procedures with a new antimicrobial surgical glove. Surg Infect (Larchmt). 2014 Feb;15(1):43-9. DOI: 10.1089/sur.2012.230
[21] Daeschlein G, Kramer A, Arnold A, Ladwig A, Seabrook GR, Edmiston CE Jr. Evaluation of an innovative antimicrobial surgical glove technology to reduce the risk of microbial passage following intraoperative perforation. Am J Infect Control. 2011 Mar;39(2):98-103. DOI: 10.1016/j.ajic.2010.05.026




