Repeated applications of cold atmospheric pressure plasma does not induce resistance in Staphylococcus aureus embedded in biofilms
Rutger Matthes 1Ojan Assadian 2
Axel Kramer 1
1 Institute of Hygiene and Environmental Medicine, University Medicine Greifswald, Germany
2 Department for Hospital Hygiene and Infection Control, Medical University of Vienna, Vienna General Hospital, Vienna, Austria
Abstract
Introduction: The increasing microbial resistance against antibiotics complicates the therapy of bacterial infections. Therefore new therapeutic options, particularly those causing no resistance, are of high interest. Cold atmospheric plasma is one possible option to eradicate multidrug resistant microorganisms, and so far no resistance development against physical plasma is known.
Method: We tested 6-fold repeated plasma applications on a Staphylococcus aureus strain embedded in biofilm and compared the reduction of the colony forming units between the different treatment periods to asses a possible development of resistance.
Result: For all treatment periods, the control biofilms were reduced by plasma in average by 1.7 log10 CFU, and decreased from 7.6 to 5.8 log10 (CFU/cm2) within 5 hours. The results demonstrated that repeated plasma doses not induce resistance or habituation against plasma applied within short time periods.
Conclusion: The repeated application of cold plasma is a promising option for the treatment of infected wounds without the risk of development of resistance against plasma.
Keywords
cold atmospheric pressure plasma, resistance development
Introduction
The development of bacterial resistance against antibiotics is an increasing challenge especially in health care [1], [2], [3]. In most situations, the microbial colonization of abiotic and biotic surfaces is accompanied with biofilm formation, which is an important pathogenic factor and one reason for direct or indirect support of the development of bacterial resistance [4]. Therefore, new therapeutic options to inactivate or to remove biofilms are of high interest.
Biofilms inhibit or block wound healing [5], [6]. In this context it is promising that the application of cold atmospheric argon plasma by the plasma source kinpen09 [7] is effective not only against biofilms [8], but induced complete healing of chronic wounds which did not respond to conventional and surgical treatment measures [9], [10].
The antimicrobial effect of plasma against a wide spectrum of bacteria including antibiotic resistant strains have been studied and reported in a number of experiments on solid agar plates [11], [12] and biofilms [13], [14], [15]. Currently, development of bacterial resistance against plasma is unknown and not expected, as its antimicrobial mode of action is physical and unspecific [16], [17]. The main target is the bacterial cell wall or membrane, which reacts with oxygen and nitrogen species in the plasma flow or in ambient liquid, resulting in lipid and protein oxidation or metabolic disruption [18]. However, plasma can modulate stress responses of microorganisms, which was demonstrated for Bacillus subtilis [19]. This may indicate the possibility for a potential bacterial habituation against physical plasma. Therefore, we investigated the influence of 6 repeated application steps of argon plasma on Staphylococcus aureus embedded in biofilms.
Methods
Microbial cultivation
The test organism Staphylococcus aureus ATCC 6538 was incubated for 48 h at 37°C on polystyrene in a 96-well-microplate (Techno Plastic Products AG, Trasadingen, Switzerland). The growth medium was similar to an artificial wound medium (minimal essential medium with 10% fetal bovine serum; GIBCO-Invitrogen, Darmstadt, Germany) [20], [21]. 80 µl of the inoculum at a concentration of 108 CFU/mL was used for four resp. five wells in 6 separate microplates for plasma treatment and control specimen. Additionally, a negative control was carried along, which was not treated with plasma at any time period. Before plasma application, the biofilms were washed with 90 µL Dulbecco’s buffered saline solution (PAA Laboratories/GE Healthcare Europe GmbH, Munich, Germany) one time.
Plasma treatment
A spatial afterglow cold plasma was generated by a radio frequency plasma pen (kinpen09®, neoplas GmbH, Greifswald, Germany) [7], using argon (99.995% pure) as carrier gas with a controlled gas flow rate at 5 sLm (standard litre/min) (MKS Instruments, Munich, Germany). The input power was set at 1.1 MHz at 2–6 kVpp with a maximal input DC power of 3.5 W to the hand-held unit, resulting in a mean heat output of approximately 300 mW on the target surface [7].
During the treatment the generated plasma jet was directed at the treated surface open to the indoor air. For all experiments, the plasma pen was fixed in a computer-controlled x/y/z table (modified EDX-20, Roland DG, Westerlo, Belgium) above the biofilm containing microplate [9]. The distance between the nozzle of the plasma pen and the biofilm was 10 mm. After 20 sec plasma treatment of each biofilm and each microplate, 80 µl medium was transferred in all biofilm-containing wells and incubated again for 1 h at 37°C.
All 6 microplates prepared with biofilm were plasma-treated. A separate plate with 5 biofilm wells without treatment served as negative control and further 5 biofilm wells served as control to check the stability of the plasma efficacy between the first and the last plasma exposure. A final separate plate served as plasma control to control for possible changes in plasma efficacy, and control biofilms for the first CFU assay of the biofilms in microplate #1 at beginning of the experiments. After 1 hour of incubation of the remaining 5 biofilm prepared microplates, all biofilms were plasma-treated, with exception of the control biofilms on the microplate #2 at time 1 hour. The same procedure was performed for the positive plasma controls. This procedure was repeatedly performed 6 times in sequential duplicates (n=9). At time 6 hour, all biofilm-covered wells were treated by plasma, again, with exception of the negative control (Figure 1 [Fig. 1]).
Figure 1: Treatment regimen of the 6 biofilm prepared 96-well-microplates for each treatment period. Note plasma treated control biofilms in black and plasma treated biofilms in red letters, blue microplates were used to determine the colony forming units (CFU). 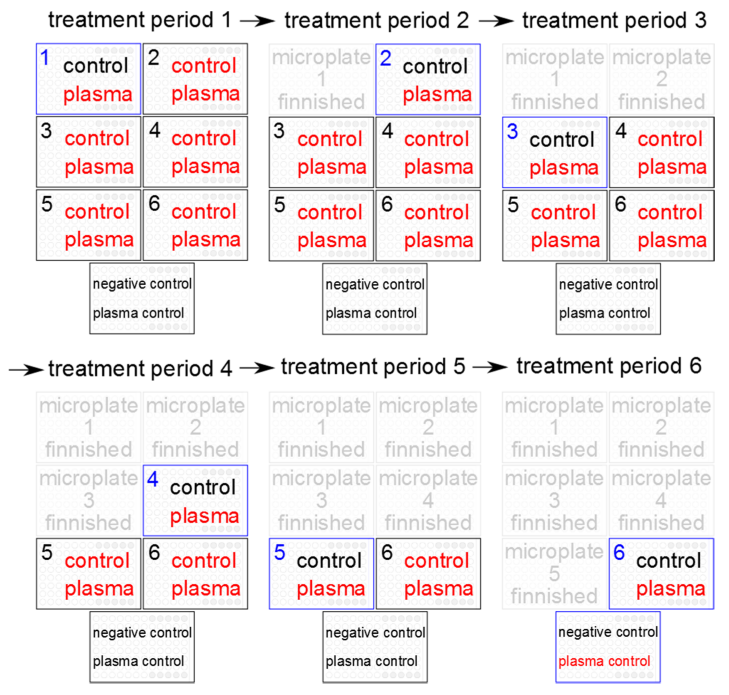
Analysis
After exposure, biofilms were dispersed in an ultrasonic bath (130 W, Branson 2510, Emerson Technologies GmbH & Co. OHG, Dietzenbach, Germany) for 20 min. The antimicrobial effect was determined as the difference in the number of CFU in the suspension as described before [14]. The CFU of the treated sample (vs) were compared with the mean of the non-treated control sample (mc) of each test run. The reduction factor (RF) was defined by the formula:
The standard deviations (±) and p values (α=0.05) were calculated based on the RFs in log10 (CFU). Statistical differences were analyzed using the Kruskal-Wallis test, followed by the Dunn’s Multiple Comparison Test (Prism, GraphPad, USA).
Results and discussion
This is the first study investigating a potential resistant development in Gram psositive bacteria embedded in biofilm. The analysis was performed after 0, 1, 2, 3, 5 and 6 hour. For all treatment periods, the control biofilms were reduced by plasma in average by 1.7 log10 CFU, and decreased from 7.6 to 5.8 log10 (CFU/cm2) within 5 hours (Figure 2 [Fig. 2]). The comparison of the RFs at times 0, 1 and 3 h showed statistically significant differences compared to the RF after the 6th hour (p<0.05). The negative control (mean 7.7 ±0.4 log10) and the positive plasma control (RF 0.8±0.4) significant differences to their initial inoculums at start (p<0.05). Conclusively, the plasma efficiency was constant for the 6 treatment periods.
Figure 2: Mean values of the CFU of S. aureus ATCC 6538 of the control biofilms (grey bars) and of the reduction factors after argon plasma treatment (dark blue bars) of each treatment period after 0, 1, 2, 3, 5 and 6 h; error bars show the standard deviation (each n=9).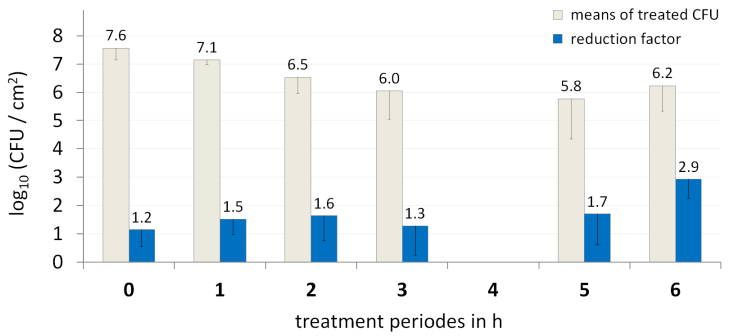
At every hour, the bacteria cells could replicate themselves and had the theoretical possibility to adapt to the plasma-induced stress. By comparing the RF of each treatment period, a difference of the susceptibility against plasma should be observable. After 4 times of plasma treatment (3rd hour) the replication-rate and the reduction-rate due to plasma were kept a level of approximately 6 log10 (CFU/cm2). Within the 6 times of hourly plasma treatment no decreased susceptibility of S. aureus against the antimicrobial components and the stress caused by 60 sec of argon plasma treatment was observable. After the 6th treatment the resistance against plasma decreased slightly, but not significantly. However, the reduction factor increased trendwise, while not significantly. Thus, a “gradual habituation” to the plasma reactive compound was not detectable in this experiment for the investigated S. aureus strain.
Conclusively, a repeated plasma application with a stable reduction-rate over time is expected. These results are in concordance with another study where the study group tested the possible bacterial resistance against plasma treatment on Escherichia coli and Enterococcus mundtii. There, induction of resistance against plasma was also not detectable [22].
A further detail of interest was that it was demonstrated that plasma has no remanent antibacterial effect. To ensure an antimicrobial long term effect to avoid a microbial recovery, the plasma treatment could be combined with antiseptics with remanent efficacy, such as used for the treatment of chronic wounds with application of polihexanide or octenidine after plasma treatment [9], [10], [23].
Conclusion
The antimicrobial effect of plasma on S. aureus is stable for repeated application doses. Since no induction of bacterial resistance against plasma treatment was observed, this method may be an option for the treatment of infected wounds. Because the antimicrobial effect shows no remanent effect, a repeated application seems to be required or a combination with topical antiseptics. Repeated plasma applications for a higher number and longer time period should be investigated in further studies.
Notes
Competing interests
The authors declare that they have no competing interests.
Funding source and acknowledgements
This study was conducted within the multi-disciplinary cooperative research program “Campus PlasmaMed”, in particular within the sub-project “PlasmaCure”, and was supported by a grant from the German Ministry of Education and Research (BMBF, grant No. 13N11181).
References
[1] Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J Jr; Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008 Jan;46(2):155-64. DOI: 10.1086/524891[2] Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119-46. DOI: 10.1146/annurev.biochem.78.082907.145923
[3] Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 2010 Apr;8(4):260-71. DOI: 10.1038/nrmicro2319
[4] Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005 Jan;13(1):34-40. DOI: 10.1016/j.tim.2004.11.010
[5] Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008 Jan-Feb;16(1):2-10. DOI: 10.1111/j.1524-475X.2007.00283.x
[6] Burmølle M, Thomsen TR, Fazli M, Dige I, Christensen L, Homøe P, Tvede M, Nyvad B, Tolker-Nielsen T, Givskov M, Moser C, Kirketerp-Møller K, Johansen HK, Høiby N, Jensen PØ, Sørensen SJ, Bjarnsholt T. Biofilms in chronic infections – a matter of opportunity – monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol. 2010 Aug;59(3):324-36. DOI: 10.1111/j.1574-695X.2010.00714.x
[7] Weltmann KD, Kindel E, Brandenburg R, Meyer C, Bussiahn R, Wilke C, von Woedtke T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib Plasma Physics. 2009;49(9):631-40. DOI: 10.1002/ctpp.200910067
[8] Matthes R, Koban I, Bender C, Masur K, Kindel E, Weltmann KD, Kocher T, Kramer A, Hübner NO. Antimicrobial Efficacy of an Atmospheric Pressure Plasma Jet Against Biofilms of Pseudomonas aeruginosa and Staphylococcus epidermidis. Plasma Process Polym. 2013 Feb;10(2):161-6. DOI: 10.1002/ppap.201100133
[9] Bender C, Hübner NO, Weltmann KD, Scharf C, Kramer A. Tissue tolerable plasma and polihexanide: Are synergistic effects possible to promote healing of chronic wounds? In vivo and in vitro results. In: Machala Z, Hensel K, Akishev Y, editors. Plasma for Bio-Decontamination, Medicine and Food Security. Dordrecht: Springer; 2012. p. 321-34. NATO Science for Peace and Security Series A: Chemistry and Biology). DOI: 10.1007/978-94-007-2852-3_25
[10] Kramer A, Lademann J, Bender C, Sckell A, Hartmann B, Münch S, Hinz P, Ekkernkamp A, Matthes R, Koban I, Partecke I, Heidecke CD, Masur K, Reuter S, Weltmann KD, Koch S, Assadian O. Suitability of Tissue Tolerable Plasmas (TTP) for the management of chronic wounds. Clin Plasma Med. 2013 Jun;1(1):11-8. DOI: 10.1016/j.cpme.2013.03.002
[11] Daeschlein G, von Woedtke T, Kindel E, Brandenburg R, Weltmann KD, Jünger M. Antibacterial Activity of an Atmospheric Pressure Plasma Jet Against Relevant Wound Pathogens in vitro on a Simulated Wound Environment. Plasma Process Polym. 2010 Mar;7(3-4):224-30. DOI: 10.1002/ppap.200900059
[12] Daeschlein G, Scholz S, Arnold A, Podewils von S, Haase H, Emmert S, von Woedtke T, Weltmann KD, Jünger M. In Vitro Susceptibility of Important Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process Polym. 2012 Apr; 9(4):380-9. DOI: 10.1002/ppap.201100160
[13] Koban I, Matthes R, Hübner N, Welk A, Meisel P, Holtfreter B, Sietmann R, Kindel E, Weltmann KD, Kramer A, Kocher T. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J Phys. 2010 Jul;12(7):073039. DOI: 10.1088/1367-2630/12/7/073039
[14] Hübner NO, Matthes R, Koban I, Rändler C, Müller G, Bender C, Kindel E, Kocher T, Kramer A. Efficacy of chlorhexidine, polihexanide and tissue-tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin Pharmacol Physiol. 2010;23(suppl 1):28-34. DOI: 10.1159/000318265
[15] Matthes R, Bender C, Schlüter R, Koban I, Bussiahn R, Reuter S, Lademann J, Weltmann KD, Kramer A. Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS ONE. 2013;8(7):e70462. DOI: 10.1371/journal.pone.0070462
[16] Helmke A, Hoffmeister D, Berge F, Emmert S, Laspe P, Mertens N, Vioel W, Weltmann KD. Physical and Microbiological Characterisation of Staphylococcus epidermidis Inactivation by Dielectric Barrier Discharge Plasma. Plasma Process Polym. 2011 Apr;8(4):278-86. DOI: 10.1002/ppap.201000168
[17] Wu H, Sun P, Feng H, Zhou H, Wang R, Liang Y, Lu J, Zhu W, Zhang J, Fang J. Reactive Oxygen Species in a Non-thermal Plasma Microjet and Water System: Generation, Conversion, and Contributions to Bacteria Inactivation – An Analysis by Electron Spin Resonance Spectroscopy. Plasma Process Polym. 2012 Apr;9(4):417-24. DOI: 10.1002/ppap.201100065
[18] Dobrynin D, Fridman G, Friedman G, Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys. 2009 Nov; 11(11):115020. DOI: 10.1088/1367-2630/11/11/115020
[19] Winter T, Winter J, Polak M, Kusch K, Mäder U, Sietmann R, Ehlbeck J, van Hijum S, Weltmann KD, Hecker M, Kusch H. Characterization of the global impact of low temperature gas plasma on vegetative microorganisms. Proteomics. 2011 Sep;11(17):3518-30. DOI: 10.1002/pmic.201000637
[20] Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008 Jun;61(6):1281-7. DOI: 10.1093/jac/dkn125
[21] Matthes R, Bender C, Schlüter R, Koban I, Bussiahn R, Reuter S, Lademann J, Weltmann KD, Kramer A. Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS ONE. 2013;8(7):e70462. DOI: 10.1371/journal.pone.0070462
[22] Zimmermann JL, Shimizu T, Schmidt HU, Li YF, Morfill GE, Isbary G. Test for bacterial resistance build-up against plasma treatment. New J Phys. 2012;14(7):073037. DOI: 10.1088/1367-2630/14/7/073037
[23] Bender C, Kramer A. Wundheilungsförderung durch kombinierte Anwendung von Tissue Tolerable Plasma und Antiseptika: Fallbeispiele aus der Veterinärmedizin. 11. Kongress für Krankenhaushygiene; 2012 Mar 25-28; Berlin. Hyg Med. 2012;01(37 Suppl):27-8.




